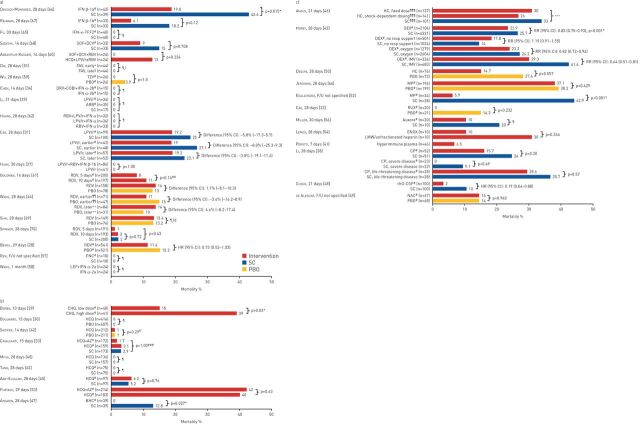
FIGURE 2.
Summary of mortality outcomes in trials of a) antivirals, b) antimalarial and mucolytic drugs and c) other therapies included in the qualitative synthesis. Other therapies include anti-inflammatory drugs, anticoagulants, kinase inhibitors, calcium release-activated calcium channel inhibitors, anticoagulants, immunomodulatory therapies and repair therapies. Results from one study are not presented graphically. Deftereos et al. [36] reported event-free survival as a primary outcome, which was defined as survival without meeting the primary clinical end-point (deterioration by 2 points on a 7-grade clinical status scale, ranging from able to resume normal activities to death). IFN: interferon; SC: standard care; TFF2: trefoil factor 2; SOF: sofosbuvir; DCV: daclatasvir; RBV: ribavirin; HCQ: hydroxychloroquine; LPV/r: lopinavir/ritonavir; FAV: favipiravir; TZV: triazavirin; PBO: placebo; DRV: darunavir; COBI: cobicistat; ARB: arbidol; RDV: remdesivir; F/U: follow-up; FNC: azvudine; LEF: leflunomide; CHQ: chloroquine diphosphate; AZ: azithromycin; BHC: bromhexine; HC: hydrocortisone; DEX: dexamethasone; IMV: invasive mechanical ventilation; MP: methylprednisolone; RUX: ruxolitinib; ENOX: enoxaparin; LMW: low molecular weight; CP: convalescent plasma; rhG-CSF: recombinant human granulocyte colony-stimulating factor; NAC: N-acetylcysteine. #: treatment administered in addition to SC, as defined by the investigators in each trial; ¶: no between-group comparison; +: treatment on day 1 of study participation; §: treatment on day 6 of study participation; ƒ: outcome was disease progression or death; ##: p-value for comparison of 7-level ordinal outcome (including death) at 14 days; ¶¶: treatment ≤10 days of symptom onset; ++: treatment >10 days of symptom onset; §§: re-analysis of data from Wang et al. [44] using different criteria; ƒƒ: outcome was incidence of hospitalisation or death; ###: p-value for comparisons of the 7-level ordinal outcome (including death) at 15 days; ¶¶¶: participants could be randomly assigned to other interventions within other therapeutic domains; +++: median (95% CI) adjusted odds ratios versus the no-hydrocortisone group were 1.03 (0.53–1.95) and 1.10 (0.58–2.11) for the fixed-dose and shock-dependent dosing hydrocortisone groups, respectively. These yielded 54% and 62% Bayesian posterior probabilities of superiority. *: statistically significant p-value.