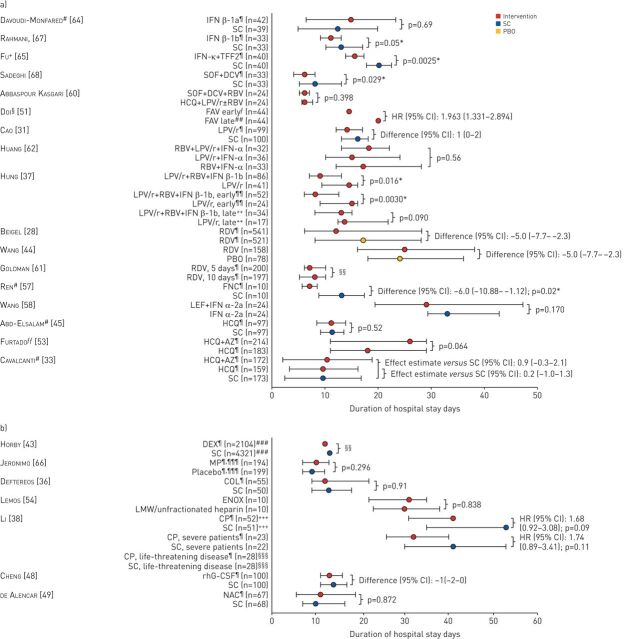
FIGURE 3.
Duration of hospitalisation in trials of a) antivirals, antimalarial and mucolytic drugs and b) other therapies included in the qualitative synthesis. Data are presented as median (IQR) unless indicated otherwise. Other therapies include anti-inflammatory drugs, anticoagulants, immunomodulatory therapies and repair therapies. IFN: interferon; SC: standard care; TFF2: trefoil factor 2; SOF: sofosbuvir; DCV: daclatasvir; RBV: ribavirin; HCQ: hydroxychloroquine; LPV/r: lopinavir/ritonavir; FAV: favipiravir; RDV: remdesivir; PBO: placebo; FNC: azvudine; LEF: leflunomide; AZ: azithromycin; DEX: dexamethasone; MP: methylprednisolone; COL: colchicine; ENOX: enoxaparin; LMW: low molecular weight; CP: convalescent plasma; rhG-CSF: recombinant human granulocyte colony-stimulating factor; NAC: N-acetylcysteine. #: mean±sd; ¶: treatment administered in addition to SC, as defined by the investigators in each trial; +: mean (95% CI); §: post-hoc analysis; ƒ: treatment on day 1 of study participation; ##: treatment on day 6 of study participation; ¶¶: treatment <7 days from symptom onset; ++: treatment ≥7 days from symptom onset; §§: no between-group comparison; ƒƒ: outcome only assessed in survivors; ###: no IQR reported; ¶¶¶: per hospital protocol, all patients meeting acute respiratory distress syndrome criteria were given pre-emptively intravenous ceftriaxone (1 g twice for 7 days) plus azithromycin (500 mg once for 5 days) or clarithromycin (500 mg twice for 7 days), starting on day 1; +++: upper IQR limit could not be determined; §§§: median value could not be determined, HR 1.90 (95% CI 0.45–8.04); p=0.38. *: statistically significant p-value.