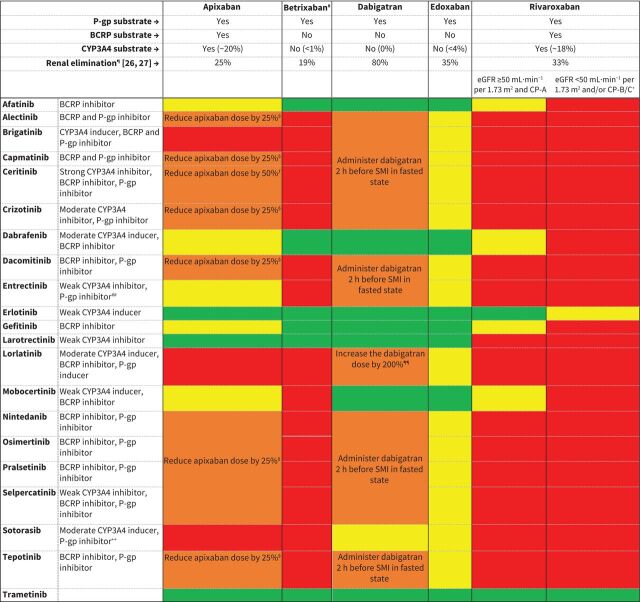
FIGURE 1.
Recommendations for combining direct oral anticoagulants (DOACs) and small-molecule inhibitors (SMIs) and dose adjustments of DOACs (classification based on table 1). BCRP: breast cancer resistance protein; CP-A/B/C: Child–Pugh A/B/C; CYP3A4: cytochrome P450 isoenzyme 3A4; dabigatran: dabigatran-etexilate; eGFR: estimated glomerular filtration rate; P-gp: P-glycoprotein. #: only available in the USA. ¶: percentage of dose excreted in urine as unchanged drug. +: in patients with renal or hepatic impairment (eGFR <50 mL·min−1 per 1.73 m2 and/or CP-B/C), concomitant use of inhibitors of P-gp, BCRP or CYP3A4 could result in an extensive increase in exposure of rivaroxaban and hence, bleeding risk [28]; thus, extra caution is warranted. §: reduce apixaban dose by 25% with P-gp inhibitors: 5 mg twice daily → 5 mg morning and 2.5 mg afternoon/10 mg twice daily → 7.5 mg twice daily. ƒ: reduce apixaban dose by 50% with strong CYP3A4 inhibitors, e.g. ceritinib: 5 mg twice daily → 2.5 mg twice daily/10 mg twice daily → 5 mg twice daily. ##: entrectinib increases exposure to digoxin, a sensitive P-gp substrate, by only 18%; therefore, no dose reduction of apixaban with entrectinib is warranted (yellow). ¶¶: increase dabigatran dose by 200%: 150 mg twice daily → 450 mg twice daily/110 mg twice daily → 330 mg twice daily. ++: sotorasib increases exposure to digoxin, a sensitive P-gp substrate, by only 21%; therefore, separation of administration of dabigatran with sotorasib is not needed (yellow).