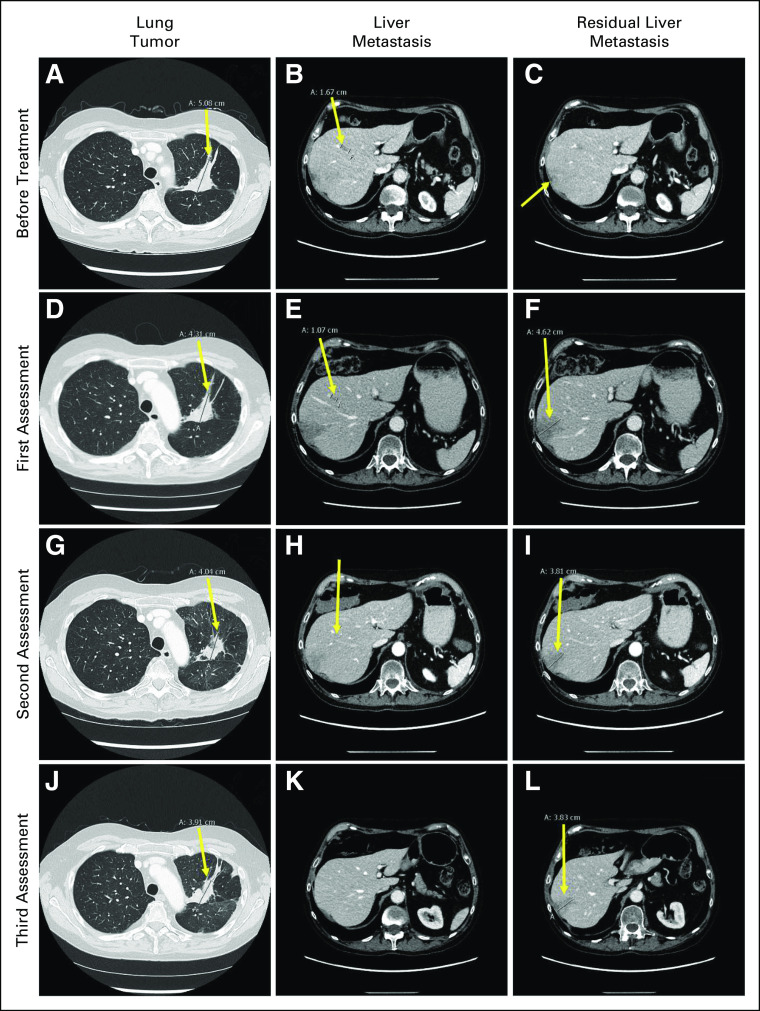
FIG 3.
Response to combined treatment with osimertinib and pralsetinib. Before treatment: (A) lung tumor in left upper lobe (5.1 cm; arrow), (B) liver metastasis (1.7 cm; arrow), and (C) residual liver metastasis (nonmeasurable; arrow). First assessment: (D) lung tumor in left upper lobe (4.3 cm; arrow), (E) liver metastasis (1.1 cm; arrow), and (F) residual liver metastasis (4.6 cm; arrow). Second assessment: (G) lung tumor in left upper lobe (4.0 cm; arrow), (H) liver metastasis (nonmeasurable; arrow), and (I) residual liver metastasis (3.8 cm; arrow). Third assessment: (J) lung tumor in left upper lobe (3.9 cm; arrow), (K) liver metastasis (nonvisible), and (L) residual liver metastasis (3.8 cm; arrow).