Abstract
According to an evolutionist approach, laughter is a multifaceted behaviour affecting social, emotional, motor and speech functions. Albeit previous studies have suggested that high-frequency electrical stimulation (HF-ES) of the pregenual anterior cingulate cortex (pACC) may induce bursts of laughter—suggesting a crucial contribution of this region to the cortical control of this behaviour—the complex nature of laughter implies that outward connections from the pACC may reach and affect a complex network of frontal and limbic regions. Here, we studied the effective connectivity of the pACC by analysing the cortico-cortical evoked potentials elicited by single-pulse electrical stimulation of pACC sites whose HF-ES elicited laughter in 12 patients. Once these regions were identified, we studied their clinical response to HF-ES, to reveal the specific functional target of pACC representation of laughter. Results reveal that the neural representation of laughter in the pACC interacts with several frontal and limbic regions, including cingulate, orbitofrontal, medial prefrontal and anterior insular regions—involved in interoception, emotion, social reward and motor behaviour. These results offer neuroscientific support to the evolutionist approach to laughter, providing a possible mechanistic explanation of the interplay between this behaviour and emotion regulation, speech production and social interactions.
This article is part of the theme issue ‘Cracking the laugh code: laughter through the lens of biology, psychology and neuroscience’.
Keywords: stereo-electroencephalography, electrical stimulation, effective connectivity, emotional mirroring, emotion regulation
1. Introduction
Laughter represents a long-lasting and yet unsolved issue for neuroscientists. Traditionally, studies on the neural basis of laughter were primarily driven by clinical interests, laughter being a distinctive sign of different pathological conditions pertaining to brain lesions or epilepsy (see [1]). Such studies, focused on the pathological production of laughter, put in the spotlight the role of subcortical structures (e.g. hypothalamus, brainstem) in generating the motor pattern of laughter. This view was well-matched with mainstream psychological theories of laughter, which considered laughter as a peripheral motor output triggered by more interesting cognitive antecedents, such as humour appreciation, sense of superiority or cognitive incongruence [2].
Laughter, however, is not only a mere subcortical phenomenon. According to an emerging evolutionary social–functional account, laughter is a multifaceted social behaviour actively contributing to the reinforcement of ongoing interactions, affiliation and communicative intents [3–8]. It carries information on the behavioural intentions of the agent, and the identity and hierarchical position of the recipient. In addition, following a fortunate perspective initiated by James [9], the physical act of laughing, along with its interoceptive feedback, is conceived to be a quintessential element in the constitution of our perceived sense of happiness which, in turn, downregulates social anxiety and negative emotions [10–12]. Interpreting laughter as a genuine socio-emotional complex behaviour, rather than a peripheral consequence of humour appreciation, makes a case for its complex cerebral representation, moving beyond subcortical structures and potentially encompassing several regions of the social and emotional brain.
In the recent past, studies conducted in surgical patients demonstrated that laughter can be elicited from the pregenual anterior cingulate cortex (pACC) by using high-frequency electrical stimulation (HF-ES; [13–18]). In these studies, the motor act of laughter was often accompanied by a sense of merriment, along with autonomic responses and interoceptive sensations [14,15,17,18]. These findings suggest that the pregenual sector of the ACC (pACC) subfield contributing to laughter production (hereafter, pACC-L for brevity) may control both the motor and the emotional aspects of laughter, in line with William James' theory [19]. The emotional interpretation of pACC-L laughter is also substantiated by imaging studies—showing that this region is structurally and functionally associated with subjective happiness [20]—and tractography studies—showing descending connections from pACC-L to the ventral striatum [21], a key reward centre whose stimulation also elicits mirthful laughter [22,23]. However, whether pACC-L controls the motor act of laughter independently of the voluntary motor system is still unclear.
Concerning the link between pACC-L, emotional laughter and social cognition, we recently reported that the same pACC sector eliciting bursts of laughter when stimulated is also activated by the passive observation of others’ laughter [16]. This finding is in accord with the contribution of the anterior cingulate cortex to the facial mimicry of dynamic positive expressions [24], and leads to the hypothesis that the pACC-L hosts an emotional mirror system boosting laughter contagion [25–27].
The aim of the present study is to deepen our understanding of the pACC-L by investigating its outward connectivity to other cortical areas. A first experimental question concerns the hypothesis that pACC-L controls emotional laughter independently of the recruitment of the motor regions controlling voluntary laughter, namely the primary motor and premotor cortices [28]. Indeed, the lack of projections towards these regions would make a case for the involvement of the pACC-L in the control of emotional, but not voluntary, laughter. A second experimental question is whether pACC-L connectivity is more in line with classic accounts linking laughter to humour processing, or with a socio-emotional account. If laughter is primarily triggered by cognitive aspects of humour appreciation—as suggested by classic psychological theories—one would expect the existence of pACC-L connections with regions associated with humour processing, such as the middle and superior temporal gyrus and the temporo–occipital–parietal junction [29–33]. By contrast, if laughter is primarily a social behaviour boosting affiliation during positive, playful social situations—as suggested by the socio-emotional account of laughter—one would expect predominant projections from the pACC-L to regions encoding social reward, interoception and the affective aspects of social interaction, such as the anterior insula, the orbitofrontal cortex (OFC) and the anterior cingulate [34–36].
The interplay between the pACC-L and other cortical functions has been investigated by combining two distinct advantages of electrical stimulation in drug-resistant epileptic patients undergoing stereo-electroencephalography (SEEG) investigation. First, we analysed cortico-cortical evoked potentials (CCEPs) elicited by single-pulse electrical stimulation (SPES) of the pACC-L, and traced the effective connectivity of this cingulate sector. This technique allowed us to reveal the causal influence that the pACC-L exerts over other cortical regions with an unmatched spatio-temporal resolution. Subsequently, we studied the effect of HF-ES applied to the sites showing effective connectivity with the pACC-L.
2. Material and methods
(a) Patient selection
We included in the study patients who underwent SEEG at the ‘Claudio Munari’ Center for Epilepsy Surgery, Niguarda Hospital, Milan, Italy, starting from May 1996, and who met the following criteria: (a) availability of anatomical and clinical data, including HF-ES and SPES, and (b) location of at least one site in the anterior cingulate cortex whose HF-ES elicited laughter or smiling responses (see below). We excluded patients whose seizure onset zone (SOZ) was in the anterior cingulate cortex. Twelve patients (L = 7, R = 5) met these criteria. Sites were mainly located in the pACC and in adjacent regions (figure 1; electronic supplementary material, figure S1).
Figure 1.
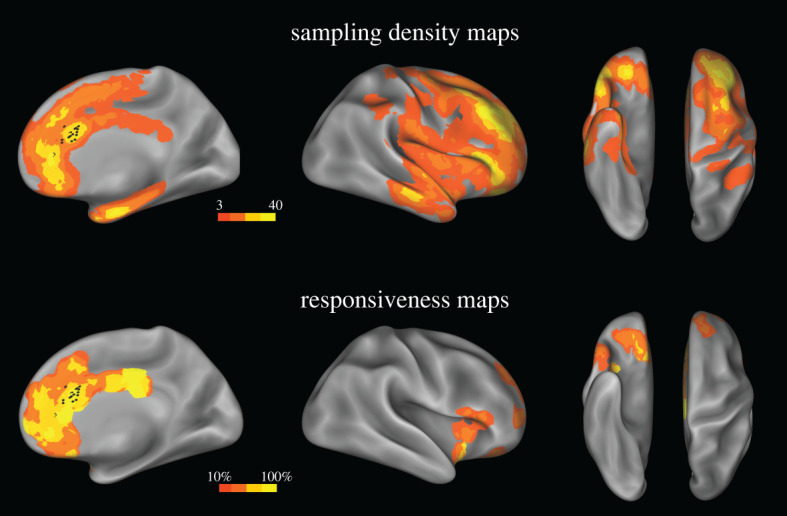
Sampling density and responsiveness maps. Upper panel. The site sampling density is shown on the inflated surface of the FS_LR brain template. The colour scale indicates the number of contacts within a disc of 1 cm radius centred on each node of the mesh. Black dots indicate the contacts whose HF-ES induced laughter and whose SPES was used for the CCEP study. Lower panel. The proportion of responsive contacts out of the overall number of stimulated contacts is plotted on the FS_LR brain template. The colour scale indicates the percentage of responsive contacts within a disc 1 cm in radius centred on each node of the mesh. Both left and right contacts are plotted on the right hemisphere. (Online version in colour.)
All patients, or their guardians, gave their informed consent to the surgical procedure and to the reviewing of data for scientific purposes. The present study received the approval of the Ethical Committee of Niguarda Hospital (ID 348-24.06.2020).
(i) . Description of the laughter response
The laughter-inducing effects of HF-ES in the present cohort have been described in detail in previous publications from our group [14–16], but given that this result is an important inclusion criterion of the present study—and a core element for the interpretation of other data—here we will briefly summarize their morphology.
Facial display. The facial component of laughter induced by HF-ES of the pACC-L typically begins with contraction of the zygomatic muscle contralateral to the stimulated hemisphere and subsequently involves the lower and upper facial muscles. Vocalization. Vocalizations or exhalations of air were observed in most cases, although their presence was not systematic. Interaction with speech. When performed during speech (e.g. reading aloud, counting, answering questions, naming months; see also §2d, below), pACC-L stimulation altered the rhythm of speech in one case, but speech arrest or speech impairments were very rare, or absent. Shift from laughter to smile. Overt bursts of laughter involving vocalizations and postural movements were co-localized with mild smiles, and occasionally obtained from the same patients/sites, on different stimulations, by simply decreasing the current intensity. Stimulation at rest. When tested at rest (e.g. patient alone in the room, not speaking), HF-ES produced a milder but clear production of the same expressions. Subjective report. In most cases, patients verbally reported having an uncontrollable and inexplicable urge to laugh, accompanied by a sense of cheerfulness associated with a perceived tendency to laugh. When asked explicitly to justify their behaviour, patients either gave post hoc justifications or admitted that they were unable to explain the reason for their behaviour.
Three patients of the present cohort were also enrolled in the study by Caruana et al. [16], demonstrating that the same pACC sites whose HF-ES elicited laughter and smiling were also selectively activated by the passive observation of dynamic videos depicting actors simulating laughter (with crying and negative expressions as control). Finally, two patients had never been published before.
(b) . Electrode implantation and contact localization
SEEG electrodes were implanted only for clinical purposes. The hemisphere investigated, the location and the number of sites were based on hypotheses about the SOZ-derived clinical history and examination, non-invasive long-term video-EEG monitoring, and neuroimaging [37,38]. Each subject underwent brain MRI (Achieva 1.5T, Philips Healthcare) and CT (O-arm 1000 system, Medtronic) to acquire appropriate sequences for SEEG planning. The duration of SEEG investigation was based only on clinical needs. Placement of intracerebral electrodes was performed under general anaesthesia by means of a robotized passive tool-holder (Neuromate, Renishaw Mayfield SA). A variable number of platinum–iridium semi-flexible multi-contact intracerebral electrodes with a diameter of 0.8 mm, a contact length of 2 mm, an inter-contact distance of 1.5 mm and a maximum of 18 contacts per electrode (Microdeep intracerebral electrodes, D08, Dixi Medical) were placed and fixed. After implantation, a fine cone-beam CT dataset was acquired by using the O-arm and coregistered with the T1-weighted three-dimensional magnetic resonance image to verify the actual position of the electrodes. The anatomical reconstruction procedure has been described in previous studies from our group [39,40]. Finally, to assess the distribution of our sampling over the cortical sheet, we identified the exact location of each recording site according to the Lausanne2008 parcellation (resolution 60; [41]). See electronic supplementary material, figure S2 template, which subdivides the entire brain into 129 different cortical and subcortical structures [42].
(c) . Single-pulse electrical stimulation procedure and intracerebral recording
SEEG signals were recorded using a 192-channel recording system (Nihon Kohden Neurofax-1200) with a sampling rate of 1000 Hz. Recordings were referenced to a contact located entirely in white matter. During invasive diagnostic evaluation, patients underwent spontaneous EEG recording in wakefulness/sleep and SPES was performed during eyes-open resting wakefulness [43,44]. SPES was performed to identify eloquent areas and effective networks connected with the SOZ [45–47]. SPES was delivered through each pair of adjacent contacts, by means of biphasic rectangular stimuli of alternating polarity (frequency: 1 Hz; pulse width: 0.5 ms; duration: 15 s; current intensity: 5 mA).
(d) . High-frequency electrical stimulation procedure
After the recording of spontaneous seizures, HF-ES was performed via electrodes in many cerebral structures, aimed at both inducing seizures and at brain mapping. Bipolar HF-ES of pairs of adjacent contacts was carried out by means of biphasic rectangular stimuli of alternating polarity (frequency: 50 Hz; pulse width: 0.5–1 ms; duration: 5 s; current intensity: up to 3 mA). Stimulations were delivered while patients were maintaining the Mingazzini position and speaking aloud, to evaluate upper limb movements, speech arrest and other behavioural modifications. All the elicited responses were video-recorded and prospectively stored in clinical report documents. All behavioural responses were assessed by two expert neurologists during the stimulation procedure.
As previously mentioned, this study was carried out in a cohort of 12 patients for whom HF-ES of the pACC successfully elicited laughter or smiling. In this study, laughter induced by HF-ES of the pACC-L was an inclusion criterion (see §2a(i)) and was not investigated further. By contrast, here we will report the results of HF-ES applied to the sites showing effective connectivity (revealed by CCEPs, see below) with the pACC-L, characterizing the type of clinical response according to the following categories: motor behaviour, speech impairments, interoceptive/emotional manifestations, somatosensory manifestations, visual/auditory events, and other responses.
(e) . Pre-processing of spontaneous recordings
Data were imported from EEG Nihon Kohden format into Matlab (MathWorks) and converted using a customized Matlab-based script. Data underwent linear detrending and high-pass filtering (0.5 Hz, third-order Butterworth filter, zero-phase shift). Bipolar montages were calculated by subtracting the signals from adjacent contacts of the same depth-electrode to minimize volume conduction and to maximize spatial resolution [48]. Data were visually inspected by trained neurophysiologists, and contacts exhibiting sustained artefactual activity or continuous epileptiform SEEG activity were excluded from further analysis to avoid interference of non-biological and pathological activity with the subsequent quantifications. Contacts used for the physiological investigation underwent further visual inspection in order to mark and remove electrical artefacts and possible, rare interictal epileptic activity.
(f) . Pre-processing of cortico-cortical evoked potentials evoked by single-pulse electrical stimulation and effective connectivity evaluation
During the long-term invasive-EEG monitoring, the effective connectivity of the explored areas was assessed for each and every subject by evaluating the CCEPs elicited by SPES [44,47], as in Russo et al. [49]. First, CCEPs were re-referenced to bipolar reference (i.e. adjacent contacts of the same depth-electrode were subtracted as in the pre-processing of the spontaneous activity. Electrical stimulation artefacts were removed by applying a Tukey-windowed median filter. Signals were then filtered (0.5 Hz high-pass third-order Butterworth filter) and single trials were split based on the inter-stimulus interval (−330 ms, +666 ms). Each trial was baseline-corrected (from 300 to 20 ms before the pulse) to avoid possible stimulation artefact residuals. SPESs delivered with alternate monophasic pulses were analysed independently between the two polarities. We performed an automatic trial rejection as in Russo et al. [49] to exclude trials affected by large epileptiform abnormalities and electrical artefacts from further analyses. After the automatic trial rejection, we categorized the receiving contacts as non-responding or connected, considering a maximum absolute voltage greater than 5σ [44,50]. As for the time window (5–150 ms), we set the onset at 5 ms because the Tukey filter used to remove possible stimulation artefacts could interfere with the data up to that point [51]. The offset at 150 ms was to avoid the classification of contacts being contaminated by the recruitment of epileptic networks, which are known to evoke a pathological complex that peaks around 200 ms [45,52–54], and to avoid the possible epileptic responses that can occur after those latencies. The automatic categorization of each contact was visually verified and corrected by two trained neurophysiologists. Subsequently, for CCEPs of each connected contact, we quantified the peak latency, as the latency of the maximum rectified peak.
3. Results
(a) . Cortico-cortical evoked potentials
(i) . Localization
Recordings were obtained from 1469 recording contacts (R = 532, L = 937) located in the cortical grey matter. The sampling density maps computed for the two hemispheres (figure 1; electronic supplementary material, figure S2; see [15,39]) show the recording coverage of the cortical sheet, with high densities of contacts located bilaterally in the frontal (63%), temporal (24%), parietal (9%) and insular (4%) cortices.
A total of 269 (R = 62, L = 207) recording contacts showed CCEP responses following SPES of the anterior cingulate cortex. These contacts were mainly located in the frontal cortex and the insula, where CCEPs were recorded in more than 20% of the overall number of contacts (27% and 20%, respectively). SPES showed CCEPs only in a few temporal contacts (3%) and never elicited significant CCEPs in the parietal contacts.
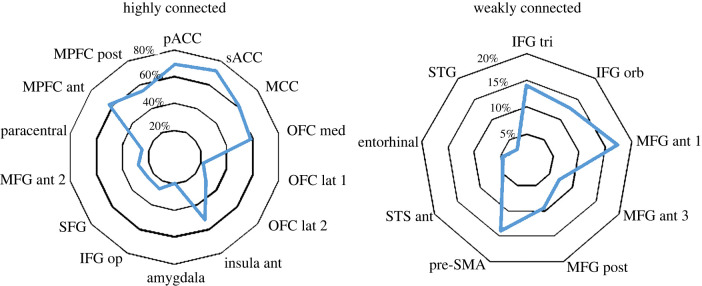
Regions where SPES of the anterior cingulate cortex elicited CCEPs in at least 20% of the recording contacts (high effective connectivity; figure 2 and table 1) include cortical territories adjacent to the stimulated sites, such as the pregenual and subgenual ACC (pACC and sACC), where SPES elicited CCEPs in about 70% of cases, the adjacent medial prefrontal cortex (MPFC), and the midcingulate cortex (MCC). More interestingly, a high percentage of CCEPs were also found in more distant cortical regions, and in particular in the medial and lateral aspects of the OFC, and the anterior insula, along with the adjacent pars opercularis of the inferior frontal gyrus (IFG op). In the lateral prefrontal cortex, significant connections were restricted to a limited sector situated between the superior and the middle frontal gyrus (SFG and MFG, respectively), despite both SFG and MFG being extensively sampled in our dataset. For all reported regions, CCEP responses were obtained from at least two patients. The amygdala—which was explored only in three patients—also showed CCEPs in 20% of contacts, albeit results were obtained only from one patient.
Figure 2.
Percentage distribution of CCEPs. Radar plots illustrate the distribution of CCEPs in regions showing high (i.e. CCEPs greater than 20% of sampled responses) and weak (i.e. CCEPs less than 20% of sampled responses) effective connectivity with the pACC. Values are expressed as percentages. sACC, subgenual ACC; pACC, pregenual ACC; MPFC, medial prefrontal cortex; MCC, midcingulate cortex; OFC, orbitofrontal cortex; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; SFG, superior frontal gyrus; STS, superior temporal sulcus; STG, superior temporal gyrus; pre-SMA, pre-supplementary motor area. For the precise location of the region according to the Lausanne2008 (resolution 60) parcellation, see electronic supplementary material, figure S2. (Online version in colour.)
Table 1.
Cortico-cortical evoked potentials. The table illustrates the list of regions showing CCEPs in at least 20% of SPES, the number of contacts sampled in each region and the number of contacts showing above-threshold CCEPs. Numbers in parentheses indicate the number of patients from which results were collected. See electronic supplementary material, table S1 for a list of all sampled regions. ROI, region of interest; sACC, subgenual ACC; pACC, pregenual ACC; MPFC, medial prefrontal cortex; MCC, midcingulate cortex; OFC, orbitofrontal cortex; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; SFG, superior frontal gyrus.
| ROI | no. recording contacts | no. CCEPs | % |
|---|---|---|---|
| sACC | 28 (7) | 20 (6) | 71.4 |
| pACC | 23 (9) | 16 (8) | 69.6 |
| MPFC ant | 43 (11) | 27 (9) | 62.8 |
| MCC | 21 (6) | 13 (4) | 61.9 |
| OFC med | 53 (12) | 31 (8) | 58.5 |
| MPFC post | 51 (9) | 28 (7) | 54.9 |
| insula ant | 25 (9) | 13 (2) | 52.0 |
| OFC lat_2 | 47 (8) | 14 (4) | 29.8 |
| MFG ant_2 | 36 (10) | 10 (2) | 27.8 |
| IFG op | 45 (11) | 12 (3) | 26.7 |
| SFG | 60 (11) | 15 (5) | 25.0 |
| paracentral | 12 (5) | 3 (2) | 25.0 |
| OFC lat_1 | 23 (6) | 5 (4) | 21.7 |
| amygdala | 15 (3) | 3 (1) | 20.0 |
The reason why some contacts/patients failed to show CCEPs—including in regions with a high connectivity—could be explained by the fact that the positions of the contacts, despite being in the same area, are not exactly overlapping in different subjects. Since the SEEG contact is extremely limited in the recording volume, it could be ineffective to record a CCEP, since it is a local field and not a far field.
To account for the possible under-sampling of some specific regions (e.g. the amygdala, which is rarely implanted in patients with implantations covering the anterior cingulate), we compared our results with the Functional Brain Tractography Project f-tract (https://f-tract.eu/; [44,55]), reporting large-scale human brain connectivity maps based on CCEPs recorded from several hundreds of patients. While providing a more extensive coverage of the cortical sheet, f-tract data were collected only on a purely anatomical basis and regardless of their responsivity to HF-ES. Notwithstanding this discrepancy, f-tract results (obtained using the Lausanne2008—resolution 60—template from the caudal anterior cingulate region) gave results comparable to ours (see electronic supplementary material, figure S3). Hence, this comparison was crucial not only because it supports our findings, but—considering that laughter typically occurs in a low percentage of pACC contacts—also because it suggests that the connectivity of pACC sites where laugher is elicited is similar to that of pACC sites where laugher is not elicited (see [56] for an evaluation of the spread of current around the electrodes when using bipolar stimulation with intensities comparable to ours).
Weak effective connectivity (i.e. responsivity <20% of the sampled contacts; figure 2) was found with the inferior frontal gyrus (pars orbitalis and triangularis), the posterior part of the MFG, the mesial aspect of the SFG bordering the pre-supplementary motor area (pre-SMA), and the anterior part of the superior temporal sulcus and gyrus (STS/STG) (see electronic supplementary material, table S1 for a complete list of the sampled regions and the connected sites).
(ii) . Peak latency
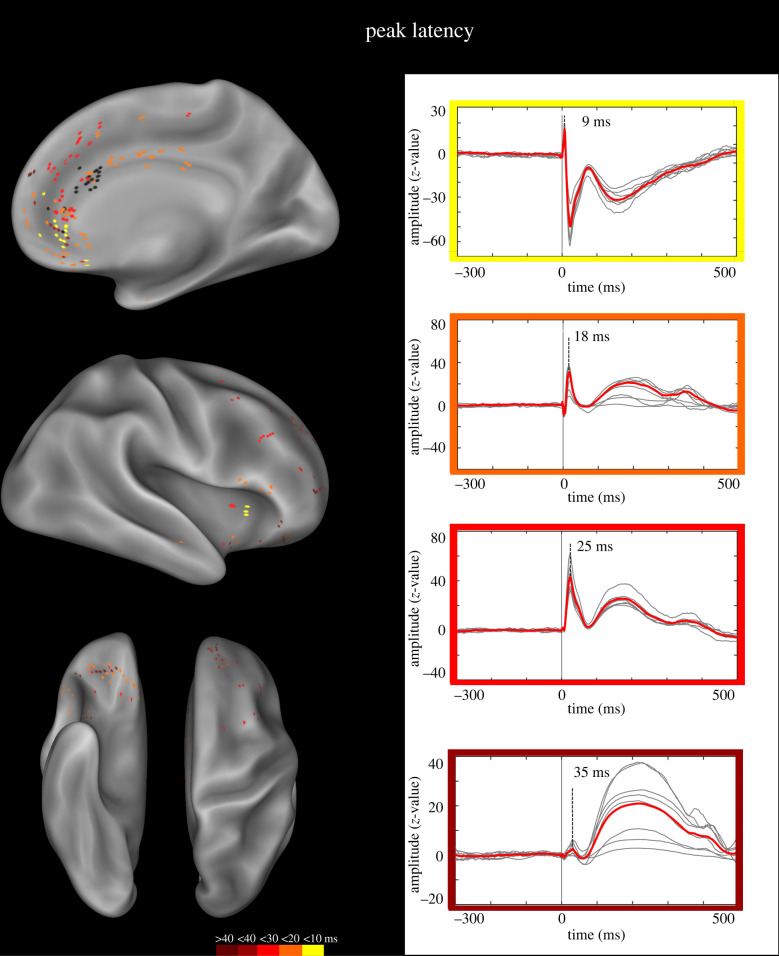
Latency of responses was defined at the peak of the first CCEP component (N1), measured according to Matsumoto et al. [43]. The peak latency of N1, collected from 223 recording contacts, ranged from 8 to 50 ms (mean 19.5 ± 8.4; figure 3). Early latencies (less than 10 ms) were predominant in the sACC, bordering the OFC and the adjacent MPFC. Of note, similar latencies were also found in the anterior insula, suggesting that (a) the latency was not linearly correlated with the distance between the pACC-L and the recording contacts, and (b) early latencies were mostly found in regions related to interoceptive/emotional functions (see §3b(i) and Discussion). Latencies in the temporal window 10–20 ms were predominantly confined to the cingulate cortex, spanning from the anteriormost cingulate sector to the MCC. Outside the cingulate cortex, similar latencies were recorded from contacts in the OFC, MPFC and IFG. Latencies greater than 20 ms were predominant outside the cingulate cortex, and particularly dense in the MPFC and lateral prefrontal cortex (SFG and MFG).
Figure 3.
N1 peak latency. Left panel. Anatomical distribution of the peak latency of the N1 potential. The colour scale indicates the CCEP peak latency. Black dots indicate the contacts whose HF-ES induced laughter and whose SPES was used for the CCEP study. Both left and right contacts are plotted on the right hemisphere of the inflated surface of the FS_LR brain template. Right panel. Representative CCEP for each of four temporal windows (CCEPs with latency greater than 40 ms not shown). (Online version in colour.)
(b) . High-frequency electrical stimulation of the target sites
Once the effective connectivity of pACC-L sites had been assessed by CCEPs, we investigated the effect of HF-ES applied to the contacts connected to the pACC-L. HF-ESs were collected from 164 (R = 40, L = 124) out of 269 connected contacts. This number is compatible with the fact that HF-ES is typically performed by choosing only one pair of contacts among all those exploring a specific anatomical structure. All the structures showing CCEPs were stimulated by HF-ES. More specifically, stimulated sites covered large sectors of the frontal lobe, including the cingulate cortex (sACC, pACC and MCC), prefrontal cortex (MPFC, SFG, MFG, IFG), OFC and pre-SMA. Outside the frontal lobe, data from the anterior insula, the amygdala and the superior temporal gyrus were also collected (table 2). Behavioural responses or subjectively reported manifestations were elicited in 43.3% of all contacts (R = 13, L = 58), while the remaining 56.7% of contacts (R = 27, L = 66) were unresponsive to electrical stimulation.
Table 2.
High-frequency electrical stimulations. The table illustrates the list of regions from which HF-ESs were collected, the number of stimulated and responsive contacts, and the distribution of the elicited responses over the seven categories: mot., motor behaviour; som., somatosensory manifestations; int., interoceptive/emotional manifestations; spe., speech impairments; vis., visual; aud., auditory; oth., other responses. All other abbreviations as in figure 2.
| ROI | HF-ES | resp. | per cent | mot. | som. | int. | spe. | vis. | aud. | oth. |
|---|---|---|---|---|---|---|---|---|---|---|
| sACC | 11 | 4 | 36.4 | 0 | 0 | 3 | 1 | 0 | 0 | 0 |
| pACC | 13 | 10 | 76.9 | 5 | 6 | 2 | 2 | 0 | 0 | 0 |
| MPFC ant | 15 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MCC | 12 | 10 | 83.3 | 2 | 7 | 0 | 0 | 1 | 0 | 0 |
| OFC med | 26 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MPFC post | 14 | 7 | 50.0 | 5 | 1 | 0 | 6 | 0 | 0 | 1 |
| insula ant | 10 | 8 | 80 | 0 | 2 | 6 | 0 | 0 | 0 | 0 |
| OFC lat_2 | 11 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MFG ant_2 | 5 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IFG op | 4 | 2 | 50.0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 |
| SFG | 8 | 7 | 87.5 | 3 | 0 | 0 | 2 | 0 | 0 | 2 |
| paracentral lobule | 3 | 3 | 100.0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 |
| OFC lat_1 | 3 | 1 | 33.3 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| amygdala | 2 | 2 | 100.0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| MFG ant_1 | 7 | 2 | 28.6 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| IFG triangularis | 5 | 5 | 100.0 | 0 | 1 | 2 | 3 | 0 | 0 | 2 |
| pre-SMA | 4 | 4 | 100.0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| IFG orb | 3 | 2 | 66.7 | 0 | 0 | 2 | 2 | 0 | 0 | 0 |
| MFG post | 5 | 2 | 40.0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 |
| STG | 2 | 2 | 100.0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| MFG ant_3 | 1 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
(i) . Motor, interoceptive and sensory responses elicited by high-frequency electrical stimulations
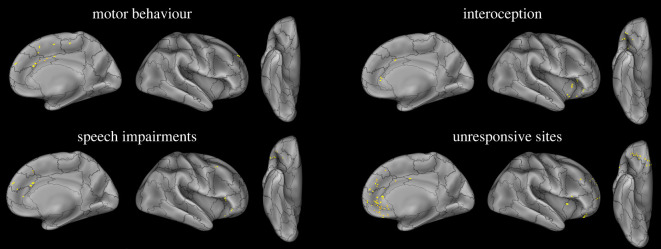
Responses elicited by HF-ES of regions connected to the pACC-L (i.e. sites showing CCEPs following SPES of the pACC-L) were classified according to general categories similar to the ones employed in Caruana et al. [15]: motor behaviour, speech impairments, interoceptive/emotional manifestations, somatosensory manifestations, visual, auditory and other responses (figure 4 and table S2; electronic supplementary material, figure S4).
Figure 4.
Responses elicited by HF-ES. Anatomical distribution of the contacts whose stimulation elicits behavioural and subjective responses belonging to the main categories of response, and unresponsive contacts. Both left and right contacts are plotted on the right hemisphere of the inflated surface of the FS_LR brain template. (Online version in colour.)
Motor behaviours were elicited in 32.4% (R = 4, L = 19) of responsive contacts, located in the MCC, the posterior aspect of the MPFC and the SFG/pre-SMA (figure 4 and table 2). Elicited responses included complex movements of the contralateral hand and upper limb and, to a lesser extent, contralateral versive head and eye movements. None of the elicited behaviours consisted of facial expressions, including smiling or laughter. In the superior frontal gyrus bordering the pre-SMA (SFG/pre-SMA), such movements were also occasionally associated with a prolonged, unemotional vocalization.
Speech impairments were elicited in 29.6% (R = 2, L = 19) of all responsive contacts, typically by the electrical stimulation of the posterior MPFC. Occasionally, they were also elicited by stimulating the inferior and the middle frontal gyri (IFG and MFG; figure 4 and table 2). Speech impairments ranged from dysarthric speech to speech arrest.
Interoceptive/emotional manifestations and autonomic responses were elicited in 23.9% (R = 3, L = 14) of all responsive contacts, mostly from the anterior insula, the adjacent anterior sector of the IFG, and the anterior cingulate cortex (sACC and pACC; figure 4 and table 2). They typically consisted of negative valenced events such as nausea, heat, anxiety, tachycardia, redness, shortness of breath and undescribed inner symptoms.
Somatosensory manifestations were elicited in 28.2% (R = 3, L = 17) of all responsive contacts, and were predominantly elicited from the pACC and MCC, and only sporadically evoked from the IFG, anterior insula and MFG (electronic supplementary material, figure S4; table 2). Such manifestations consisted of paraesthetic symptoms affecting the upper limb or the face, and sensations of electric shock.
Finally, the stimulation of 15.5% (R = 3, L = 8) of all responsive contacts elicited visual and auditory hallucinations or undescribed responses, which were clearly perceived but difficult to define. Visual hallucinations were obtained from the posterior part of the cingulate cortex, at the level of the paracentral lobule. Auditory hallucinations were elicited from the STG. The remaining undefined effects were elicited from the SFG, IFG and amygdala (electronic supplementary material, figure S4; table 2).
(ii) . Laughter was not elicited by high-frequency electrical stimulations of pregenual anterior cingulate cortex-L connected regions
HF-ES of regions connected to the pACC-L never induced mirthful or mirthless laughter, mild smiles, emotional displays or positive emotions in any case. Moreover, in none of the elicited responses was it possible to envision any functional connection with laughter or part of it.
It can therefore be concluded that, in our dataset, the only region from where laughter was elicited by HF-ES is the pACC-L, laughter elicited by the pACC-L being an inclusion criterion of the present study (see §2a(i)). The fact that laughter was not elicited from any of the regions connected to the pACC-L rules out the possible objection that the laughter response originally elicited from the pACC-L could be due to the downstream recruitment of pACC-connected areas.
4. Discussion
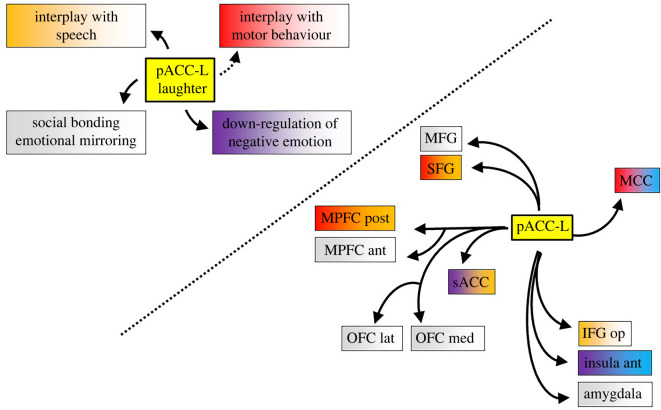
The present study stemmed from the evidence that HF-ES of the pACC elicits bursts of laughter, and investigated the anatomical and functional interactions between the pACC-L and other cortical regions. This goal was achieved by combining two distinct approaches to electrical stimulation in SEEG patients. First, the analyses of CCEPs elicited by SPES of the pACC-L revealed that the pACC-L is part of a wide network encompassing prefrontal and limbic regions but, interestingly, sparing the fronto-parietal networks for the control of voluntary motor functions. Subsequently, the study of HF-ES applied to the contacts showing effective connectivity with the pACC-L showed that neither laughter, nor part of it, was elicitable by stimulating the regions connected with the pACC-L, ruling out the hypothesis that pACC-L laughter was a side-effect due to the recruitment of downstream cortical connections. Taken together, our results suggest that the pACC-L is a crucial node of the emotional pathway for laughter, independent from the volitional pathway for laughter, targeting and possibly modulating multiple behavioural, socio-emotional and speech-related functions (figure 5). Our results and their implications are discussed in detail below.
Figure 5.
Summary of pACC projections and possible relationship with laughter functions. The interplay between pACC representation of laughter and social, emotional and motor functions (left panel) can be accounted for by the outward connectivity of the pACC towards frontal and limbic regions (right panel). The colour code is evocative of the functions derived by HF-ES and other data debated in the discussion (red, motor; orange, speech; violet, interoceptive/emotional; blue, somatosensory; grey, unresponsive). Abbreviations as in figure 2. (Online version in colour.)
(a) . The networks of laughter
In our study, the frontal/Rolandic operculum and the ventral premotor cortex, albeit well sampled, have virtually no outward connections with the pACC-L, despite their recognized role in laughter and smile production ([16,57,58]; see also [28]). This result—combined with our finding that HF-ES failed to elicit laughter in any of the sites targeted by the pACC-L—brings more grist to the mill of the assumption that the emotional pathway for laughter, originating in the pACC, and the volitional pathway, housed in the voluntary motor system, are essentially segregated, in line with previous models [1,6,21,59–61]. By contrast, a connection between the pACC-L and pre-SMA, despite the sparse sampling of the latter, is partially suggested by our findings showing a weak connectivity between the pACC-L and SFG/pre-SMA, and is in line with previous effective and structural connectivity studies [18,21].
These results are compatible with a recent tractography study [21], reporting that the brain regions whose HF-ES elicits laughter—namely the frontal/Rolandic operculum [16,57,58], the pre-SMA [62–64], the anterior temporal lobe [16,65–67], the ventral striatum [22,23] and the pACC-L [13–18]—constitute two partially segregated networks. A first network, likely involved in the production of emotional laughter, encompasses the pACC-L, the anterior temporal lobe and the ventral striatum, and a second network, involved in volitional and non-emotional laughter, is anchored to the frontal/Rolandic operculum and the primary motor cortex—with pre-SMA connected to both the pACC-L and frontal/Rolandic operculum.
The parallelism between our effective connectivity data and structural connectivity data is, by contrast, more complex when considering the connection between the pACC-L and the other cortical node of the emotional network, i.e. the anterior temporal lobe. In our study, in fact, we did not find any clear projection from the pACC-L to the anterior temporal lobe, but only a few contacts showing CCEPs in the rostralmost part of the STS. This result leads to three mutually exclusive hypotheses: (a) the representation of laughter in the temporal region is not connected to that housed in the pACC-L, despite the structural connection, (b) the temporal region responsible for laughter extends caudally, including the anterior STS (very unlikely, given that there are no reports of laughter elicited by HF-ES of the anterior STS), or (c) the anterior temporal lobe has asymmetric effective connectivity patterns, projecting to—but not receiving from—the anterior cingulate. The last hypothesis—supported by previous findings that asymmetric effective connectivity patterns characterize the temporal lobe [68]—is particularly intriguing, as it suggests that social and emotional information encoded in the anterior temporal lobe [69] is projected to the pACC-L, eventually inducing mirthful laughter.
(b) . Laughter interaction with interoceptive and emotional systems
Our study shows that SPES of the pACC-L elicited CCEPs in a wide set of regions associated with emotional and interoceptive functions, such as the subgenual and pregenual ACC (where sites inducing laughter are mostly located), the anterior insula and, to a lesser extent, the amygdala. CCEPs in these regions were particularly rapid, as shown by the fact that the majority of contacts from which early latencies (less than 10 ms) were recorded were located in the sACC and the anterior insula. Consistently with the emotional and interoceptive functions typically attributed to these regions, we found that the majority of HF-ESs eliciting interoceptive/emotional manifestations were obtained by stimulating contacts in the anterior insula and anterior cingulate sectors, in accord with previous stimulation studies [15,18,70–73]—while the stimulation of the amygdala, frequently inducing fear and vegetative responses [74,75], gave undescribed responses in one patient.
Since—with the exception of the pACC—all the above-mentioned regions have been associated with negative emotions and emotional disorders such as depression, sadness, fear and social anxiety [76–78], a particularly intriguing question is why they receive input from a cingulate field conveying positive-valenced, mirthful laughter.
A possible answer comes from studies showing that emotional laughter downregulates anxiety, stress, depression and other negative emotional states [10–12]. In line with such an interpretation, a recent electrical stimulation study [17] reported that in three patients undergoing an awake craniotomy procedure, stimulation of the dorsal anterior cingulate bundle, adjacent to the pACC-L, induced robust anxiolytic responses to the point that intravenous anaesthetic/anxiolytic medications were discontinued. Hence, it is reasonable to suppose that such connections may represent a mechanistic explanation of the modulatory role of laughter on negative emotions, a hypothesis feeding the well-known role of the ACC in emotion regulation [79–81], and its impaired regulatory function in patients with generalized social phobia or generalized anxiety disorder [82].
(c) . Laughter interactions with motor behaviour and speech production
Albeit our CCEP study clearly demonstrated a lack of direct projections to the motor/premotor cortex lying on the lateral surface of the cerebral hemisphere, we recorded CCEPs from the MCC, a cingulate region also contributing to motor behaviour, as witnessed by the HF-ES of the contacts located in this region, and by previous stimulation studies [15,83]. The functional role of such connection is challenging, in particular if considering that the responses elicited by HF-ES concerned the upper limbs, rather than mouth/face movements, as would be expected. It must be noticed, however, that the impact of laughter on our motor behaviour goes far beyond the mere control of the face muscles—involving proximal limb and axial muscles [84]—and there is evidence that laughter is a demanding exercise for trunk muscles, even more so than many other traditional exercises regarding mean trunk muscle activity [85].
Another complex motor behaviour whose interaction with the pACC-L is suggested by our results is speech. First, we found that CCEPs were systematically elicited from the IFG, a crucial node of the language network [86]. Second, our HF-ES study revealed that speech impairments were elicited not only following stimulation of the IFG (in accord with Mălîia et al. [87]), but also from other regions receiving pACC-L projections, including cortical regions not primarily involved in speech production, such as the MPFC.
The interplay between speech and laughter is indeed a non-trivial complex phenomenon. Provine [88, p. 239] noted that ‘although conversation is filled with laughter, the laughs do not occur randomly. The placement of laughter in speech is akin to punctuating written text and is termed the punctuation effect. A speaker's laughter usually occurs before and after complete statements and questions, and seldom interrupts phrase structure’. This indirect link between the pACC-L and speech impairments—also in line with the assumption that speech depends on two parallel motor systems, with vocalizations produced by an emotional motoneuronal pathway involving the cingulate cortex [60]—can well explain why, although human vocalization is rarely altered by cingulate electrical stimulation, lesion of the anterior cingulate results in mutism and decreased vocalizations [89].
(d) . Pregenual anterior cingulate cortex and orbitofrontal cortex: on laughter, social bonding and emotional mirroring
The OFC is one of the most distant regions reached by SPES of the pACC-L, comparable only to the case of the anterior insula, yet is one of the more systematically responding regions. Unfortunately, the equally systematic unresponsiveness of the OFC to HF-ES prevents us from using high-frequency stimulation data to unravel the possible functional role of this connection.
A recent hypothesis, mainly derived from imaging studies, is particularly relevant to our study of laughter, as it suggests that the OFC encodes others' laughter, and in particular the rewarding value of laughter and smiles expressed by familiar individuals. Capitalizing on the evidence that the OFC differentiates the sight of one's own smiling baby from the sight of an unknown smiling baby [90], Niedenthal et al. [36] suggested that the OFC distinguishes the basic properties of others' smiles from the specific reward conveyed by smiles made by people with whom we have strong affiliative relationships—and the emerging role of the OFC in evaluating rewarding affiliative smiling and laughter has been further substantiated by new studies by Kringelbach and coworkers, specifically devoted to the mother–infant relationship (see, among others, [91,92]).
How can this information help in interpreting the projection from the pACC-L (controlling one's own laughter) to the OFC (processing the affiliative value of others' laughter)? In a recent study, we demonstrated in three patients—also enrolled in the present investigation—that the pACC-L sites involved in laughter production are also active during the passive observation of dynamic videos depicting actors simulating laughter [16]. This finding—along with the evidence that the anterior cingulate contributes to the facial mimicry of positive-valenced expressions [24]—suggests that the pACC-L implements an emotional mirroring process, which potentially contributes to social bonding through emotional contagion [25–27]. Hence, it is reasonable to interpret our result of a projection from the pACC-L to the OFC by assuming that it conveys the outcome of an emotional mirroring process occurring in the pACC-L, informing the OFC about others' mirthful emotional laughter, and eventually facilitating the establishment or consolidation of social bonds. Incidentally, this hypothesis is also coherent with a more general model of the interplay between the ACC and OFC, assuming that the contribution of the former is closely bound to action representation while the latter is more engaged in the rewarding values of those actions [93]—hence placing the phenomenon of emotional mirroring within a broader framework.
5. Conclusion
Despite the predominant psychological theories of laughter regarding this behaviour as a peripheral motor output essentially pertaining to subcortical circuits, here we report that the outward connections of the pACC sector involved in the production of mirthful laughter reach a high number of cortical regions. Connected regions include the adjacent cingulate and medial prefrontal cortices, the OFC and the anterior insula, contributing to interoception, emotion, social reward and motor behaviour. Of note, the pACC-L effective connectivity spares both motor and premotor regions—confirming that the pACC-L controls emotional laughter independently from the voluntary motor system—and temporal regions encoding humour—supporting the independence of laughter from humour appreciation. These results offer neuroscientific support to the evolutionary socio-emotional theory of laughter, providing a possible mechanistic explanation of the interplay between this behaviour and emotion regulation, speech production and social interactions.
Acknowledgements
We thank Olivier David for technical support with the Functional Brain Tractography Project f-tract (https://f-tract.eu/).
Ethics
The present study received the approval of the Ethical Committee of Niguarda Hospital (ID 348-24.06.2020).
Data accessibility
The conditions of our ethics approval do not permit public archiving of individual anonymized raw data. Readers seeking access to the data should contact the corresponding author. Access will be granted to named individuals in accordance with ethical procedures governing the reuse of sensitive data. Specifically, requestors must sign a formal agreement confirming that (a) the user may not use the database for any non-academic purpose, and (b) the document must be signed by a person with a permanent position at an academic institute or publicly funded research institute. Up to five other researchers affiliated with the same institute for whom the signee is responsible may be named at the end of this document which will allow them to work with this dataset. (c) The user may not distribute the database or portions thereof in any way.
Authors' contributions
F.M.Z.: data curation, formal analysis, investigation, writing—review and editing; M.D.V.: methodology, supervision, writing—review and editing; S.R.: data curation, formal analysis, methodology, software; V.M.: data curation, investigation; V.P.: data curation, investigation; P.D.: data curation, investigation; I.S.: conceptualization, data curation, investigation, methodology, project administration, writing—review and editing; P.A.: funding acquisition, supervision, writing—review and editing; F.C.: project administration, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
M.D.V. was supported by European Union Horizon 2020 Framework Programme through Grant Agreement no. 785907 (Human Brain Project, SGA2) to P.A.
References
- 1.Lauterbach EC, Cummings JL, Kuppuswamy PS. 2013. Toward a more precise, clinically-informed pathophysiology of pathological laughing and crying. Neurosci. Biobehav. Rev. 37, 1893-1916. ( 10.1016/j.neubiorev.2013.03.002) [DOI] [PubMed] [Google Scholar]
- 2.Morreall J. 1987. The philosophy of laughter and humor. Albany, NY: State University of New York Press. [Google Scholar]
- 3.Provine RR. 2000. Laughter: a scientific investigation. New York, NY: Viking. [Google Scholar]
- 4.Provine RR. 2004. Laughing, tickling, and the evolution of speech and self. Curr. Dir. Psychol. Sci. 13, 215-218. ( 10.1111/j.0963-7214.2004.00311.x) [DOI] [Google Scholar]
- 5.Dunbar RIM. 2012. Bridging the bonding gap: the transition from primates to humans. Phil. Trans. R. Soc. B 367, 1837-1846. ( 10.1098/rstb.2011.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott SK, Lavan N, Chen S, McGettigan C. 2014. The social life of laughter. Trends Cogn. Sci. 18, 618-620. ( 10.1016/j.tics.2014.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood A, Niedenthal P. 2018. Developing a social functional account of laughter. Social Pers. Psychol. Compass 12, e12383. ( 10.1111/spc3.12383) [DOI] [Google Scholar]
- 8.Palagi E, Caruana F, de Waal FBM. 2022. The naturalistic approach to laughter in humans and other animals: towards a unified theory. Phil. Trans. R. Soc. B 377, 20210175. ( 10.1098/rstb.2021.0175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James W. 1884. What is an emotion? Mind 9, 188-205. ( 10.1093/mind/os-IX.34.188) [DOI] [Google Scholar]
- 10.Yim J. 2016. Therapeutic benefits of laughter in mental health: a theoretical review. Tohoku J. Exp. Med. 239, 243-249. ( 10.1620/tjem.239.243) [DOI] [PubMed] [Google Scholar]
- 11.Savage BM, Lujan HL, Thipparthi RR, DiCarlo SE. 2017. Humor, laughter, learning, and health! A brief review. Adv. Physiol. Educ. 41, 341-347. ( 10.1152/advan.00030.2017) [DOI] [PubMed] [Google Scholar]
- 12.Akimbekov NS, Razzaque MS. 2021. Laughter therapy: a humor-induced hormonal intervention to reduce stress and anxiety. Curr. Res. Physiol. 4, 135-138. ( 10.1016/j.crphys.2021.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperli F, Spinelli L, Pollo C, Seeck M. 2006. Contralateral smile and laughter, but no mirth, induced by electrical stimulation of the cingulate cortex. Epilepsia 47, 440-443. ( 10.1111/j.1528-1167.2006.00442.x) [DOI] [PubMed] [Google Scholar]
- 14.Caruana F, Avanzini P, Gozzo F, Francione S, Cardinale F, Rizzolatti G. 2015. Mirth and laughter elicited by electrical stimulation of the human anterior cingulate cortex. Cortex 71, 323-331. ( 10.1016/j.cortex.2015.07.024) [DOI] [PubMed] [Google Scholar]
- 15.Caruana F, et al. 2018. Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex. Brain 141, 3035-3051. ( 10.1093/brain/awy219) [DOI] [PubMed] [Google Scholar]
- 16.Caruana F, Avanzini P, Pelliccia V, Mariani V, Zauli F, Sartori I, Del Vecchio M, Russo GL, Rizzolatti G. 2020. Mirroring other's laughter. Cingulate, opercular and temporal contributions to laughter expression and observation. Cortex 128, 35-48. ( 10.1016/j.cortex.2020.02.023) [DOI] [PubMed] [Google Scholar]
- 17.Bijanki KR, et al. 2019. Cingulum stimulation enhances positive affect and anxiolysis to facilitate awake craniotomy. J. Clin. Invest. 129, 1152-1166. ( 10.1172/JCI120110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oane I, et al. 2020. Cingulate cortex function and multi-modal connectivity mapped using intracranial stimulation. Neuroimage 220, 117059. ( 10.1016/j.neuroimage.2020.117059) [DOI] [PubMed] [Google Scholar]
- 19.Caruana F. 2019. The integration of emotional expression and experience: a pragmatist review of recent evidence from brain stimulation. Emot. Rev. 11, 27-38. ( 10.1177/1754073917723461) [DOI] [Google Scholar]
- 20.Matsunaga M, Kawamichi H, Koike T, Yoshihara K, Yoshida Y, Takahashi HK, Nakagawa E, Sadato N. 2016. Structural and functional associations of the rostral anterior cingulate cortex with subjective happiness. Neuroimage 134, 132-141. ( 10.1016/j.neuroimage.2016.04.020) [DOI] [PubMed] [Google Scholar]
- 21.Gerbella M, Pinardi C, Di Cesare G, Rizzolatti G, Caruana F. 2021. Two neural networks for laughter: a tractography study. Cereb. Cortex 31, 899-916. ( 10.1093/cercor/bhaa264) [DOI] [PubMed] [Google Scholar]
- 22.Haq IU, et al. 2011. Smile and laughter induction and intraoperative predictors of response to deep brain stimulation for obsessive-compulsive disorder. Neuroimage 54(Suppl. 1), S247-S255. ( 10.1016/j.neuroimage.2010.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson WS, Cho S, Abulseoud OA, Gorny KR, Felmlee JP, Welker KM, Klassen BT, Min HK, Lee KH. 2016. The impact of mirth-inducing ventral striatal deep brain stimulation on functional and effective connectivity. Cereb. Cortex 27, 2183-2194. ( 10.1093/cercor/bhw074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rymarczyk K, Żurawski Ł, Jankowiak-Siuda K, Szatkowska I. 2018. Neural correlates of facial mimicry: simultaneous measurements of EMG and BOLD responses during perception of dynamic compared to static facial expressions. Front. Psychol. 9, 52. ( 10.3389/fpsyg.2018.00052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzolatti G, Caruana F. 2017. Some considerations on de Waal and Preston review. Nat. Rev. Neurosci. 18, 769. ( 10.1038/nrn.2017.139) [DOI] [PubMed] [Google Scholar]
- 26.de Waal FBM, Preston SD. 2017. Mammalian empathy: behavioural manifestations and neural basis. Nat. Rev. Neurosci. 18, 498-509. ( 10.1038/nrn.2017.72) [DOI] [PubMed] [Google Scholar]
- 27.Caruana F. 2021. Two simulation systems in the human frontal cortex? Disentangling between motor simulation and emotional mirroring using laughter. Cortex 148, 215-217. ( 10.1016/j.cortex.2021.09.011) [DOI] [PubMed] [Google Scholar]
- 28.Kern M, Bert S, Glanz O, Schulze-Bonhage A, Ball T. 2019. Human motor cortex relies on sparse and action-specific activation during laughing, smiling and speech production. Commun. Biol. 2, 118. ( 10.1038/s42003-019-0360-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goel V, Dolan RJ. 2001. The functional anatomy of humor: segregating cognitive and affective components. Nat. Neurosci. 4, 237-238. ( 10.1038/85076) [DOI] [PubMed] [Google Scholar]
- 30.Mobbs D, Greicius MD, Abdel-Azim E, Menon V, Reiss AL. 2003. Humor modulates the mesolimbic reward centers. Neuron 40, 1041-1048. ( 10.1016/S0896-6273(03)00751-7) [DOI] [PubMed] [Google Scholar]
- 31.Moran JM, Wig GS, Adams RB, Janata P, Kelley WM. 2004. Neural correlates of humor detection and appreciation. Neuroimage 21, 1055-1060. ( 10.1016/j.neuroimage.2003.10.017) [DOI] [PubMed] [Google Scholar]
- 32.Bartolo A, Benuzzi F, Nocetti L, Baraldi P, Nichelli P. 2006. Humor comprehension and appreciation: an FMRI study. J. Cogn. Neurosci. 18, 1789-1798. ( 10.1162/jocn.2006.18.11.1789) [DOI] [PubMed] [Google Scholar]
- 33.Tian F, et al. 2017. Getting the joke: insight during humor comprehension - evidence from an fMRI study. Front. Psychol. 8, 1835. ( 10.3389/fpsyg.2017.01835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craig A. 2002. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655-666. ( 10.1038/nrn894) [DOI] [PubMed] [Google Scholar]
- 35.Kringelbach ML. 2005. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 6, 691-702. ( 10.1038/nrn1747) [DOI] [PubMed] [Google Scholar]
- 36.Niedenthal PM, Mermillod M, Maringer M, Hess U. 2010. The simulation of smiles (SIMS) model: embodied simulation and the meaning of facial expression. Behav. Brain Sci. 33, 433-480. ( 10.1017/S0140525X10001445) [DOI] [PubMed] [Google Scholar]
- 37.Cossu M, Cardinale F, Castana L, Citterio A, Francione S, Tassi L, Benabid AL, Lo Russo G. 2005. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: a retrospective analysis of 215 procedures. Neurosurgery 57, 706-718. ( 10.1227/01.NEU.0000176656.33523.1e) [DOI] [PubMed] [Google Scholar]
- 38.Cardinale F, et al. 2019. Stereoelectroencephalography: retrospective analysis of 742 procedures in a single centre. Brain 142, 2688-2704. ( 10.1093/brain/awz196) [DOI] [PubMed] [Google Scholar]
- 39.Avanzini P, et al. 2016. Four-dimensional maps of the human somatosensory system. Proc. Natl Acad. Sci. USA 113, E1936-E1943. ( 10.1073/pnas.1601889113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avanzini P, Pelliccia V, Lo Russo G, Orban GA, Rizzolatti G. 2018. Multiple time courses of somatosensory responses in human cortex. Neuroimage 169, 212-226. ( 10.1016/j.neuroimage.2017.12.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daducci A, Gerhard S, Griffa A, Lemkaddem A, Cammoun L, Gigandet X, Meuli R, Hagmann P, Thiran JP. 2012. The connectome mapper: an open-source processing pipeline to map connectomes with MRI. PLoS ONE 7, e48121. ( 10.1371/journal.pone.0048121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagmann P, Kurant M, Gigandet X, Thiran P, Wedeen VJ, Meuli R, Thiran JP. 2007. Mapping human whole-brain structural networks with diffusion MRI. PLoS ONE 2, e597. ( 10.1371/journal.pone.0000597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Lüders HO. 2004. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain 127, 2316-2330. ( 10.1093/brain/awh246) [DOI] [PubMed] [Google Scholar]
- 44.Trebaul L, et al. 2018. Probabilistic functional tractography of the human cortex revisited. Neuroimage 181, 414-429. ( 10.1016/j.neuroimage.2018.07.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valentín A, Anderson M, Alarcón G, Seoane JJG, Selway R, Binnie CD, Binnie CD, Polkey CE. 2002. Responses to single pulse electrical stimulation identify epileptogenesis in the human brain in vivo. Brain 125, 1709-1718. ( 10.1093/brain/awf187) [DOI] [PubMed] [Google Scholar]
- 46.Keller CJ, Bickel S, Entz L, Ulbert I, Milham MP, Kelly C, Mehta AD. 2011. Intrinsic functional architecture predicts electrically evoked responses in the human brain. Proc. Natl Acad. Sci USA 108, 10 308-10 313. ( 10.1073/pnas.1019750108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto R, Kunieda T, Nair D. 2017. Single pulse electrical stimulation to probe functional and pathological connectivity in epilepsy. Seizure 44, 27-36. ( 10.1016/j.seizure.2016.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li G, Jiang S, Paraskevopoulou SE, Wang M, Xu Y, Wu Z, Chen L, Zhang D, Schalk G. 2018. Optimal referencing for stereo-electroencephalographic (SEEG) recordings. Neuroimage 183, 327-335. ( 10.1016/j.neuroimage.2018.08.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russo S, et al. 2021. Focal lesions induce large-scale percolation of sleep-like intracerebral activity in awake humans. Neuroimage 234, 117964. ( 10.1016/j.neuroimage.2021.117964) [DOI] [PubMed] [Google Scholar]
- 50.Silverstein BH, Asano E, Sugiura A, Sonoda M, Lee M-H, Jeong J-W. 2020. Dynamic tractography: integrating cortico-cortical evoked potentials and diffusion imaging. Neuroimage 215, 116763. ( 10.1016/j.neuroimage.2020.116763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pigorini A, et al. 2015. Bistability breaks-off deterministic responses to intracortical stimulation during non-REM sleep. Neuroimage 112, 105-113. ( 10.1016/j.neuroimage.2015.02.056) [DOI] [PubMed] [Google Scholar]
- 52.Valentín A, Alarcón G, Honavar M, García Seoane JJ, Selway RP, Polkey CE, Binnie CD. 2005. Single pulse electrical stimulation for identification of structural abnormalities and prediction of seizure outcome after epilepsy surgery: a prospective study. Lancet Neurol. 4, 718-726. ( 10.1016/S1474-4422(05)70200-3) [DOI] [PubMed] [Google Scholar]
- 53.Valentín A, Alarcón G, García-Seoane JJ, Lacruz ME, Nayak SD, Honavar M, Selway RP, Binnie CD, Polkey CE. 2005. Single-pulse electrical stimulation identifies epileptogenic frontal cortex in the human brain. Neurology 65, 426-435. ( 10.1212/01.wnl.0000171340.73078.c1) [DOI] [PubMed] [Google Scholar]
- 54.Kokkinos V, Alarcón G, Selway RP, Valentín A. 2013. Role of single pulse electrical stimulation (SPES) to guide electrode implantation under general anaesthesia in presurgical assessment of epilepsy. Seizure 22, 198-204. ( 10.1016/j.seizure.2012.12.012) [DOI] [PubMed] [Google Scholar]
- 55.David O, Job A-S, De Palma L, Hoffmann D, Minotti L, Kahane P. 2013. Probabilistic functional tractography of the human cortex. Neuroimage 80, 307-317. ( 10.1016/j.neuroimage.2013.05.075) [DOI] [PubMed] [Google Scholar]
- 56.Nathan SS, Sinha SR, Gordon B, Lesser RP, Thakor NV. 1993. Determination of current density distributions generated by electrical stimulation of the human cerebral cortex. Electroencephalogr. Clin. Neurophysiol. 86, 183-192. ( 10.1016/0013-4694(93)90006-H) [DOI] [PubMed] [Google Scholar]
- 57.Caruana F, Gozzo F, Pelliccia V, Cossu M, Avanzini P. 2016. Smile and laughter elicited by electrical stimulation of the frontal operculum. Neuropsychologia 89, 364-370. ( 10.1016/j.neuropsychologia.2016.07.001) [DOI] [PubMed] [Google Scholar]
- 58.Fernández-Baca Vaca G, Lüders HO, Basha MM, Miller JP. 2011. Mirth and laughter elicited during brain stimulation. Epileptic Disord. 13, 435-440. ( 10.1684/epd.2011.0480) [DOI] [PubMed] [Google Scholar]
- 59.Wild B, Rodden FA, Grodd W, Ruch W. 2003. Neural correlates of laughter and humour. Brain 126, 2121-2138. ( 10.1093/brain/awg226) [DOI] [PubMed] [Google Scholar]
- 60.Holstege G, Subramanian HH. 2016. Two different motor systems are needed to generate human speech. J. Comp. Neurol. 524, 1558-1577. ( 10.1002/cne.23898) [DOI] [PubMed] [Google Scholar]
- 61.Klingbeil J, Wawrzyniak M, Stockert A, Brandt M-L, Schneider H-R, Metelmann M, Saur D. 2021. Pathological laughter and crying: insights from lesion network-symptom-mapping. Brain 144, 3264-3276. ( 10.1093/brain/awab224) [DOI] [PubMed] [Google Scholar]
- 62.Fried I, Wilson CL, MacDonald KA, Behnke EJ. 1998. Electric current stimulates laughter. Nature 391, 650. ( 10.1038/35536) [DOI] [PubMed] [Google Scholar]
- 63.Krolak-Salmon P, Hénaff M-A, Vighetto A, Bauchet F, Bertrand O, Mauguière F, Isnard J. 2006. Experiencing and detecting happiness in humans: the role of the supplementary motor area. Ann. Neurol. 59, 196-199. ( 10.1002/ana.20706) [DOI] [PubMed] [Google Scholar]
- 64.Schmitt JJ, Janszky J, Woermann F, Tuxhorn I, Ebner A. 2006. Laughter and the mesial and lateral premotor cortex. Epilepsy Behav. 8, 773-775. ( 10.1016/j.yebeh.2006.03.003) [DOI] [PubMed] [Google Scholar]
- 65.Arroyo S, Lesser RP, Gordon B, Uematsu S, Hart J, Schwerdt P, Andreasson K, Fisher RS. 1993. Mirth, laughter and gelastic seizures. Brain 116, 757-780. ( 10.1093/brain/116.4.757) [DOI] [PubMed] [Google Scholar]
- 66.Satow T, et al. 2003. Mirth and laughter arising from human temporal cortex. J. Neurol. Neurosurg. Psychiatry 74, 1004-1005. ( 10.1136/jnnp.74.7.1004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamao Y, et al. 2015. Neural correlates of mirth and laughter: a direct electrical cortical stimulation study. Cortex 66, 134-140. ( 10.1016/j.cortex.2014.11.008) [DOI] [PubMed] [Google Scholar]
- 68.Guo Z, et al. 2021. Effective connectivity among the hippocampus, amygdala, and temporal neocortex in epilepsy patients: a cortico-cortical evoked potential study. Epilepsy Behav. 115, 107661. ( 10.1016/j.yebeh.2020.107661) [DOI] [PubMed] [Google Scholar]
- 69.Olson IR, Plotzker A, Ezzyat Y. 2007. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130, 1718-1731. ( 10.1093/brain/awm052) [DOI] [PubMed] [Google Scholar]
- 70.Isnard J, Guénot M, Sindou M, Mauguière F. 2004. Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study. Epilepsia 45, 1079-1090. ( 10.1111/j.0013-9580.2004.68903.x) [DOI] [PubMed] [Google Scholar]
- 71.Pugnaghi M, Meletti S, Castana L, Francione S, Nobili L, Mai R, Tassi L. 2011. Features of somatosensory manifestations induced by intracranial electrical stimulations of the human insula. Clin. Neurophysiol. 122, 2049-2058. ( 10.1016/j.clinph.2011.03.013) [DOI] [PubMed] [Google Scholar]
- 72.Stephani C, Fernandez-Baca Vaca G, Maciunas R, Koubeissi M, Lüders HO. 2011. Functional neuroanatomy of the insular lobe. Brain Struct. Funct. 216, 137-149. ( 10.1007/s00429-010-0296-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McLaren ME, Szymkowicz SM, O'Shea A, Woods AJ, Anton SD, Dotson VM. 2016. Dimensions of depressive symptoms and cingulate volumes in older adults. Transl. Psychiatry 6, e788. ( 10.1038/tp.2016.49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meletti S, Tassi L, Mai R, Fini N, Tassinari CA, Russo GL. 2006. Emotions induced by intracerebral electrical stimulation of the temporal lobe. Epilepsia 47, 47-51. ( 10.1111/j.1528-1167.2006.00877.x) [DOI] [PubMed] [Google Scholar]
- 75.Inman CS, Bijanki KR, Bass DI, Gross RE, Hamann S, Willie JT. 2020. Human amygdala stimulation effects on emotion physiology and emotional experience. Neuropsychologia 145, 106722. ( 10.1016/j.neuropsychologia.2018.03.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paulus MP, Stein MB. 2006. An insular view of anxiety. Biol. Psychiatry 60, 383-387. ( 10.1016/j.biopsych.2006.03.042) [DOI] [PubMed] [Google Scholar]
- 77.Stein MB, Stein DJ. 2008. Social anxiety disorder. Lancet 371, 1115-1125. ( 10.1016/S0140-6736(08)60488-2) [DOI] [PubMed] [Google Scholar]
- 78.Arias JA, et al. 2020. The neuroscience of sadness: a multidisciplinary synthesis and collaborative review. Neurosci. Biobehav. Rev. 111, 199-228. ( 10.1016/j.neubiorev.2020.01.006) [DOI] [PubMed] [Google Scholar]
- 79.Ochsner KN, Gross JJ. 2005. The cognitive control of emotion. Trends Cogn. Sci. 9, 242-249. ( 10.1016/j.tics.2005.03.010) [DOI] [PubMed] [Google Scholar]
- 80.Etkin A, Egner T, Kalisch R. 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 15, 85-93. ( 10.1016/j.tics.2010.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Etkin A, Büchel C, Gross JJ. 2015. The neural bases of emotion regulation. Nat. Rev. Neurosci. 16, 693-700. ( 10.1038/nrn4044) [DOI] [PubMed] [Google Scholar]
- 82.Blair KS, Geraci M, Smith BW, Hollon N, DeVido J, Otero M, Blair JR, Pine DS. 2012. Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol. Psychiatry 72, 476-482. ( 10.1016/j.biopsych.2012.04.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chassagnon S, Minotti L, Kremer S, Hoffmann D, Kahane P. 2008. Somatosensory, motor, and reaching/grasping responses to direct electrical stimulation of the human cingulate motor areas. J. Neurosurg. 109, 593-604. ( 10.3171/JNS/2008/109/10/0593) [DOI] [PubMed] [Google Scholar]
- 84.Cattaneo L, Pavesi G. 2014. The facial motor system. Neurosci. Biobehav. Rev. 38, 135-159. ( 10.1016/j.neubiorev.2013.11.002) [DOI] [PubMed] [Google Scholar]
- 85.Wagner H, Rehmes U, Kohle D, Puta C. 2014. Laughing: a demanding exercise for trunk muscles. J. Mot. Behav. 46, 33-37. ( 10.1080/00222895.2013.844091) [DOI] [PubMed] [Google Scholar]
- 86.Friederici AD, Gierhan SM. 2013. The language network. Curr. Opin. Neurobiol. 23, 250-254. ( 10.1016/j.conb.2012.10.002) [DOI] [PubMed] [Google Scholar]
- 87.Mălîia M-D, Donos C, Barborica A, Popa I, Ciurea J, Cinatti S, Mîndruţă I. 2018. Functional mapping and effective connectivity of the human operculum. Cortex 109, 303-321. ( 10.1016/j.cortex.2018.08.024) [DOI] [PubMed] [Google Scholar]
- 88.Provine RR. 2017. Laughter as an approach to vocal evolution: the bipedal theory. Psychon. Bull. Rev. 24, 238-244. ( 10.3758/s13423-016-1089-3) [DOI] [PubMed] [Google Scholar]
- 89.Devinsky O, Morrell MJ, Vogt BA. 1995. Contributions of anterior cingulate cortex to behaviour. Brain 118, 279-306. ( 10.1093/brain/118.1.279) [DOI] [PubMed] [Google Scholar]
- 90.Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. 2004. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage 21, 583-592. ( 10.1016/j.neuroimage.2003.10.005) [DOI] [PubMed] [Google Scholar]
- 91.Parsons CE, Young KS, Petersen MV, Jegindoe Elmholdt E-M, Vuust P, Stein A, Kringelbach ML. 2017. Duration of motherhood has incremental effects on mothers' neural processing of infant vocal cues: a neuroimaging study of women. Scient. Rep. 7, 1727. ( 10.1038/s41598-017-01776-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stark EA, Cabral J, Riem MME, Van Ijzendoorn MH, Stein A, Kringelbach ML. 2020. The power of smiling: the adult brain networks underlying learned infant emotionality. Cereb. Cortex 30, 2019-2029. ( 10.1093/cercor/bhz219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rushworth MFS, Behrens TEJ, Rudebeck PH, Walton ME. 2007. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn. Sci. 11, 168-176. ( 10.1016/j.tics.2007.01.004) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The conditions of our ethics approval do not permit public archiving of individual anonymized raw data. Readers seeking access to the data should contact the corresponding author. Access will be granted to named individuals in accordance with ethical procedures governing the reuse of sensitive data. Specifically, requestors must sign a formal agreement confirming that (a) the user may not use the database for any non-academic purpose, and (b) the document must be signed by a person with a permanent position at an academic institute or publicly funded research institute. Up to five other researchers affiliated with the same institute for whom the signee is responsible may be named at the end of this document which will allow them to work with this dataset. (c) The user may not distribute the database or portions thereof in any way.