Abstract
Background:
Despite the effectiveness of innovations to improve care of persons with dementia, there has been limited diffusion of these into widespread clinical practice. We aimed to identify common barriers and address them directly in the initial phase of dissemination of a successful dementia care program.
Methods:
Description of and early experience with a dissemination strategy of the UCLA Alzheimer’s and Dementia Care Program to health care systems nationwide. We measured site-identified goals for the program and indicators of success, number of adopting sites and participants in their programs.
Results:
From January 2019 to December 2021, 80 sites expressed interest in adopting the program, 14 (18%) sites adopted it, and 10 of these sites have begun caring for patients. Another 4 sites have implemented the program as part of a randomized clinical trial. To date, over 1690 persons living with dementia and their caregivers have received ADC care at 14 adopting sites. Key lessons from the early dissemination efforts include the importance of identifying a strong product champion at the adopting site, creating a business case for adoption, training of clinical staff, and adapting the model to fit local cultures and workflow, as well as recognizing the likely long length of time needed for the decision to adopt and implementation process.
Conclusions:
Despite many obstacles to dissemination, with local champions and technical assistance, successful innovations in dementia care can be implemented in diverse health systems. The ability of adopting sites to bring the program to full scale and achieve comparable outcomes as the original program remains to be determined.
Keywords: Alzheimer’s disease, dementia, caregiver burden, co-management, collaborative care, advance practice providers
INTRODUCTION
In the older population, Alzheimer’s disease and related dementias are common and devastating disorders for both persons affected and their caregivers. Care for dementia spans both the patient and caregiver, medical and social domains, and requires health system and community-based interventions. Early non-pharmacological interventions for dementia care focused on caregiver education and support in the context of research studies. Despite the effectiveness of numerous caregiver interventions in improving caregiver efficacy and sometimes patient outcomes, there has been little diffusion of these into widespread clinical practice,1 consistent with data demonstrating that the implementation of successful biomedical advances may take as long as 17 years.2 Dissemination of innovations to improve dementia care has been particularly difficult because of the lack of trained clinicians, the complicated nature of dementia care, and insufficient reimbursement for dementia care services.
The UCLA Alzheimer’s and Dementia Care (ADC) Program is a health system-based co-management/collaborative care model of advanced practice provider (usually Nurse Practitioners or Physician Assistants) Dementia Care Specialists (DCSs) working with primary care and specialty physicians, who focus on meeting dementia care needs.3 The program has demonstrated effectiveness on all components of the triple aim4 of better care,5 better health,6 and lower costs.7,8
In 2012, the Center for Medicare and Medicaid Innovation (CMMI) awarded a Health Care Innovations Award (HCIA) to UCLA to scale the program from 250 to 1000 local participants. The local program, which has subsequently received philanthropic and institutional support, as well as revenue from Medicare for billable services, has continued to grow at UCLA with over 3450 participants and their families having been served and over 750 currently active.
Recognizing the success of the program and widespread need for this type of care for persons living with dementia, in 2017 The John A. Hartford Foundation awarded UCLA a grant to plan for dissemination of the UCLA ADC Program. During the planning grant, UCLA identified a national nursing partner, the Gerontological Advanced Practice Nurses Association (GAPNA), to help disseminate the program; created an online continuing education curriculum for nurse practitioners to develop skills as DCSs; prepared materials for adopting sites; and began partnerships with two organizations, the Alzheimer’s Association and the American Geriatrics Society, to continue the work of dissemination. This planning grant was followed in 2018 by an implementation grant to educate nurse practitioners in dementia care management through GAPNA, to establish the UCLA ADC program in 8-10 health care systems practices nationwide, and to create a plan for more widespread implementation.
In this paper, we describe the approach to dissemination and lessons learned about barriers to adoption and keys to successful adoption. A companion paper reports the experiences of the adopting sites, including changes needed during the COVID-19 pandemic.
METHODS
This report describes dissemination efforts during the first 3 years of the implementation grant (January 1, 2019 to December 31, 2021). Dissemination of the program has been considered quality improvement by the UCLA Institutional Review Board and did not require approval. Early in the dissemination process, a decision was made to drop “UCLA” from the name of the program at adopting sites. This decision was made to recognize that the program has been modified to fit the local environment, avoid confusion among staff and patients at adopting institutions as to whose program this is, and mitigate any proprietary concerns.
We focused on three phases of dissemination: pre-adoption, implementation, and sustainability. Pre-adoption includes disseminating information about the program (marketing) and facilitating decisions to adopt. Marketing of the program included creation of a dedicated website (https://www.adcprogram.org/); inclusion on the American Geriatrics Society (AGS) website; engagement of several Alzheimer’s Association Health Systems Directors, who provide technical assistance to health systems; presentations and on-site marketing at national professional society and health care meetings and local grand rounds of interested organizations, and through direct mailings by the AGS. The ADC Program is also listed among the Best Practice Caregiving https://bpc.caregiver.org/.
Interested sites contacted UCLA and were asked to complete a brief initial interest form to describe their organization type and ownership, identify the site product champion and contact person and their role in the organization, and tell how they heard about the program. Introductory phone calls were then scheduled that included the dissemination team program director (DBR), the lead DCS (LCE), the program manager (KS), and various personnel from the potential adopting sites, including the site product champion (usually a physician or advanced practice provider) and often including other clinical staff and practice managers. These calls typically lasted 30-45 minutes during which the potential adopting site described its organization and current efforts in dementia care followed by the UCLA team briefly summarizing the ADC program and its benefits, answering questions, and outlining next steps.
After the Introductory call, organizations were sent two one-page summaries (Supplementary Description S1) describing what the implementation team could provide (e.g., modifiable clinical forms, training, technical assistance) and expectations of the adopting sites (i.e., commitment of clinical and support staff, provision of de-identified records to assess fidelity, summaries of demographic data and pre- and post-implementation clinical outcomes), and a Readiness Assessment Instrument. This instrument (Supplementary Figure S1) was short but required conversations between the product champion and site administrative leadership to identify the goals of adopting the program and local indicators of success. Garnering the support of leadership is a key step in the adoption decision and obtaining resources to implement the program. The dissemination team created a return-on-investment calculator (available by request to info@adcprogram.org), which is a workbook with spreadsheets that allows potential adopting sites to enter their own labor and start-up costs, and project the number of patients and Part B billing revenues to determine if and when the program would break even financially, and to model patient capacity and staffing needs. The was provided to the sites, upon request, after the initial call. Potential adopting sites were offered to option of having a member of the dissemination team speak to them or their leadership about the budget model and business case.
Implementation is a collaborative process between ADC dissemination staff and adopting sites that includes adapting the ADC model to the individual site; training staff, including feedback on fidelity; and providing technical assistance. Core elements of the program that must be implemented are listed in Table 1. Clinical and operational materials (e.g., pre-visit questionnaires, job descriptions, work flow, and billing information) are available on the website and the dissemination team facilitates adaptation to local environments. DCS training includes: a 22-module on-line curriculum; in-person or virtual training for skills; weekly clinical and implementation assistance including intensive 1:1 training on the DCS roles, the ADC model, and clinical cases for 8 weeks, which then decreases to monthly for up to 2 years; and weekly “office hours” with the lead UCLA DCS, which are available to adopting DCSs. Fidelity is ascertained by the dissemination team DCS’s review of 5 de-identified initial intake notes and subsequent case review of patients treated by each adoption site DCS.
Table 1.
Core Elements of Alzheimer and Dementia Care (ADC) Program
| Element (what) | Rationale (why) | Implementation Options (how) |
|---|---|---|
| Staffing | ||
| Advanced Practice Provider Dementia Care Specialist (DCS) with specific ADC training and prescribing authority | • High clinical skills • Order writing ability • Respect among physicians and staff |
• Nurse practitioner • Physician’s assistant • Clinical nurse specialist (some states) • Training modules • In-person training off- and on-site • Ongoing mentoring |
| Dementia Care Assistant | • Performs administrative duties and provides protocolized care and assistance for lower acuity patients • Allows DCS to work at top of license |
• College graduate • RN • BSW • LCSW |
| Medical Director | • Provides additional clinical expertise • Facilitates obtaining needed resources |
• Geriatrician • Neurologist • Psychiatrist |
| Program Manager | • Responsible for day-to-day operations within larger health care unit • Manages human resource and space issues |
• Existing manager (additional responsibilities) • Dedicated ADC program manager |
| Responsibilities | ||
| Longitudinal dementia care including: • Comprehensive initial and annual assessments • Continuous monitoring and assessment • Ongoing care plans • Psychosocial interventions - Aimed at person living with dementia -Aimed at caregivers • Self-management • Medication management • Treatment of related conditions • Coordination of care • Advance care planning |
• Maximizes patient function, independence, & dignity • Minimizes caregiver strain • Reduces unnecessary cost |
• Co-management • Independent dementia care practice • Primary care of patients with dementia |
| 24/7 Coverage | • Provides just-in-time problem solving • Avoids emergency department use |
• DCS-only coverage system • Coverage by another physician =/− APP coverage group |
| Infrastructure and Support | ||
| Access to EHR | • Facilitates coordination of care | • Allows for accurate documentation, billing and communication as well as measuring population reached |
| EHR modified to support dementia care work | • Improves efficiency | • Modification of existing EHR |
| Linkages to community-based services | • Expands range of services | • Referrals • Contracts |
APP=advanced practice provider
DCS=Dementia Care Specialist
EHR=electronic health record
RN=registered nurse
BSW=bachelors in social work
LCSW=licensed clinical social worker
Sustainability includes achieving organizational patient care and quality goals for the program, financial stability, and staff satisfaction. To help sites demonstrate value to their health system, after 2 years of program implementation ADC program staff will provide data on enrollment; clinical outcomes including patient behavioral symptoms and caregiver symptoms of distress, strain, depression, and burden; and utilization, including hospitalizations, ED visits, and hospice use; these results can be compared to the UCLA experience and that of other adopting sites.
Descriptive analyses were performed without testing for statistical significance because of the small sample size.
RESULTS
The flow of interest-to-adoption is presented in Figure 1. From January 2019 to December 2021, 80 sites expressed interest in adopting the program. The most common ways that sites learned about the program were: through a colleague, journal articles, the ADC website, professional meetings, and mailings from the AGS. Of the 80 sites that expressed interest, 14 (18%) sites adopted it through The John A. Hartford Foundation-funded initiative. The rate of adoption has increased during the past 3 years; 2 sites in 2019, 5 in 2020, and 7 in 2021. Another 4 sites adopted the model in 2019 as one of the intervention arms of the D-CARE study, a clinical trial comparing health systems-based versus community-based dementia care versus usual care.9 Half of adopting sites are academic health systems and the other half represent a variety of community health care systems across 10 different states (Figure 2). Four serve primarily rural communities. Their patient populations (actual or projected, depending upon start date) ranged from 0.6-52% Black and 1-23.4% Latino. Typically, the program is based in geriatrics, neurology, or palliative care but serves a wide population of patients most of whom are referred by primary care.
Figure 1.
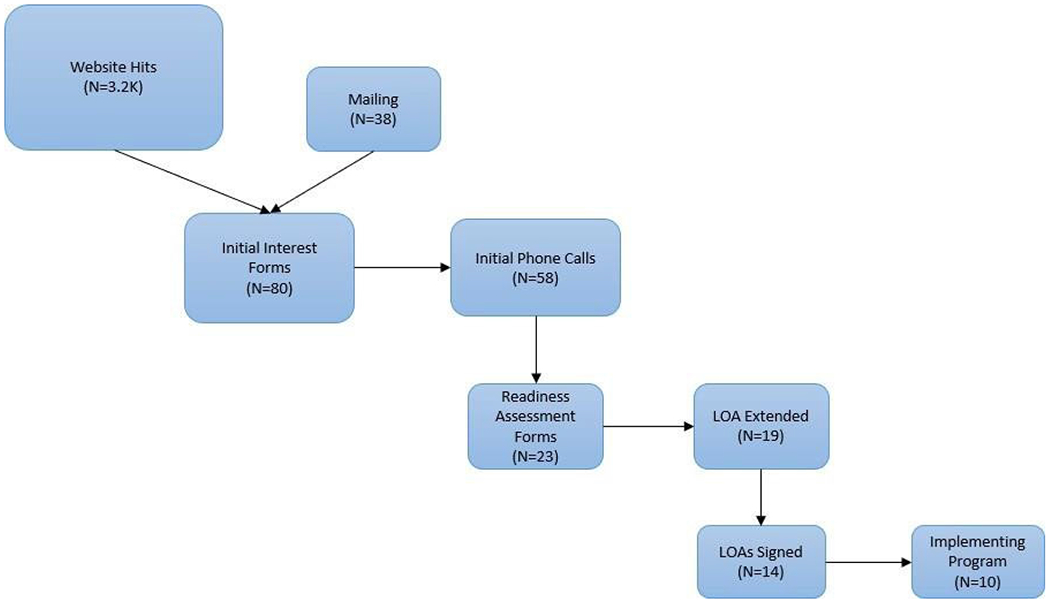
Interested and adopting sites
LOA=letter of agreement
Mailed = AGS mailed approximately 20,000 ADC Program postcards to AGS website users, both members and non-members. From that, 38 sites expressed interest and reached out to learn more about the ADC Program
Figure 2.
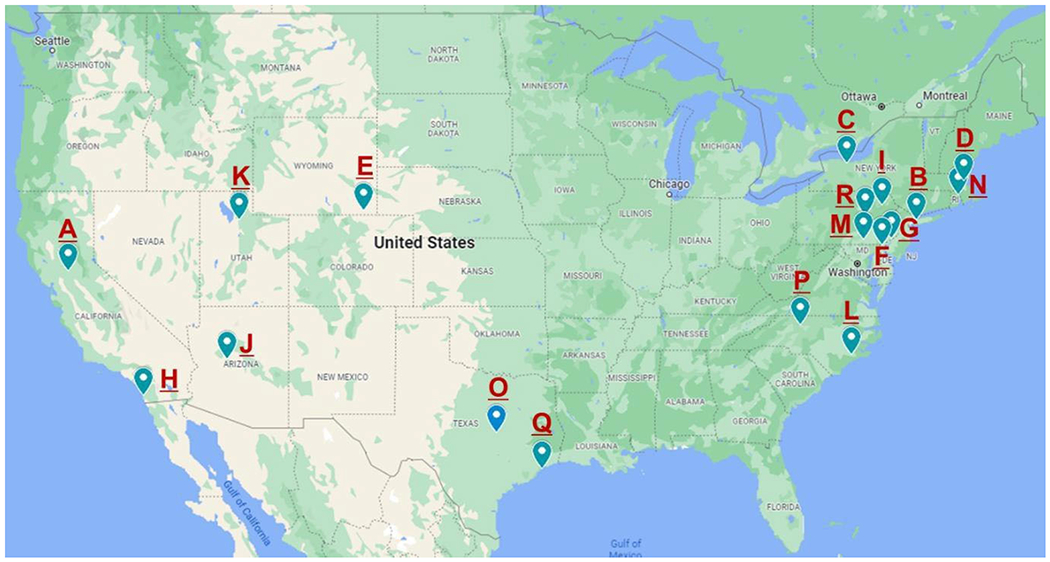
Map of adopting sites
See Supplemental Material for descriptions of sites and timelines for adoption.
The most frequently mentioned organizational goals for the program (Figure 3) were to increase dementia care coordination, improve clinical outcomes of patients, rduce caregiver stress, and reduce emergency department utilization and hospitalizations. It should be noted, however, over half of the adopting institutions rated “profit based on billing revenues” as being very important.
Figure 3.
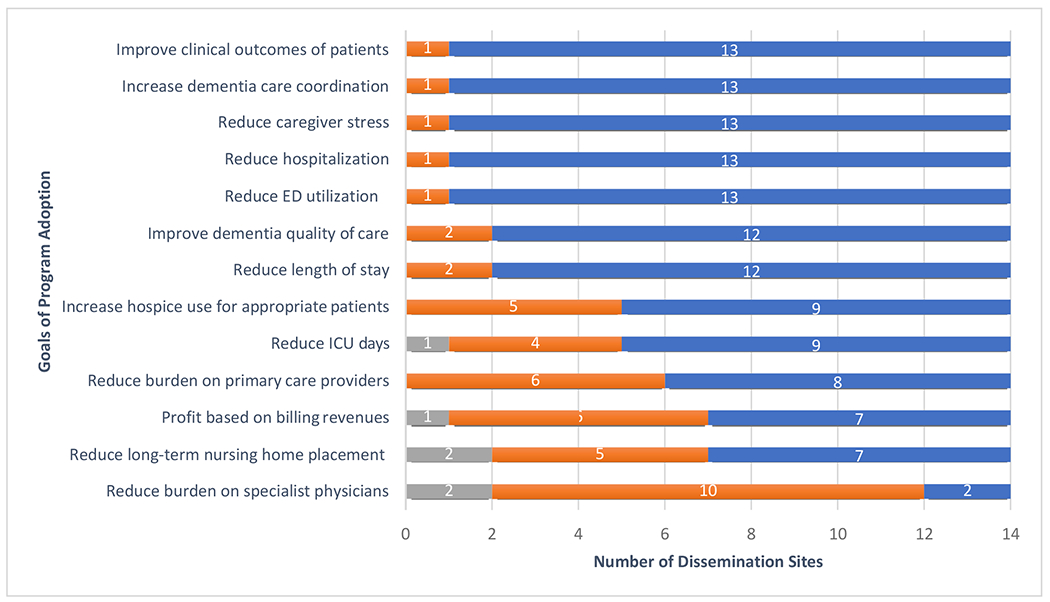
Adopting Sites’ organizational goals for the program and indicators of success
a Only JAHF adopting sites (N=14). Does not include D-CARE sites
To date, 14 of the 18 adopting sites have begun caring for patients for 1 to 14 months. These 14 sites have used 24 DCSs (21 nurse practitioners and 3 physicians’ assistants) and have cared for over 1690 persons living with dementia and their caregivers. The time from initial interest expression to first patient seen was a median time of 324 days with the largest portion (median of 124 days) between signing the letter of agreement and seeing the first patient, reflecting time needed to get the Program up and running (Supplementary Table S1).
Some of the lessons learned in the early phases of disseminating the ADC Program that should be helpful to the spread of other health care delivery innovations are presented in Table 2.
Table 2.
Lessons learned in early ADC dissemination efforts
| • Identify and nurture a product champion. Someone within the organization will need to lead the pre-adoption, implementation, and sustainability processes. |
| • The business case is critical. If local decision makers understand that program implementation will not lose large amounts of money, adoption is much more likely. |
| • Training is essential. Even highly capable clinicians need to learn content and process to provide high quality dementia care. |
| • Local factors are important in implementing the Program and defining how it and the Dementia Care Specialists relate to primary care providers and other geriatrics services. |
| • Be patient. Commitment to adoption often requires multiple decision makers, timing, and current competing priorities. |
| • Don’t underestimate the time needed. From interest to implementation with fidelity is a journey that needs assistance at every stage. |
DISCUSSION
For many reasons, the dissemination of effective innovations in health care delivery has been slow. First, communication about these innovations is usually through traditional academic routes such as presentations, which occur sporadically (e.g., national meetings of professional societies), and publications, which require writing, peer review, revision, and publication processes. Second, each of the factors guiding the decision to adopt (compatibility, complexity, trialability [i.e., the ability to try out the innovation before committing], observability, and relative advantage)10 pose variable challenges to individual health systems. For example, work flows required by the innovation and the need for electronic health record support may be incompatible with the current clinical care at an institution. Third, health delivery innovations usually require staff and infrastructure, which means either hiring new staff or redesigning existing positions. Fourth, health systems need to be able to implement the intervention with fidelity if they expect to get similar results as those achieved in traditional clinical trials, which often have research staff rather than clinical staff implementing the intervention. Other challenges in implementing clinical innovations include physical space and additional time required by clinicians (e.g., primary care providers), who may not provide the intervention but need to respond to intervention clinicians11 as well as staff turnover, departure of staff who served as champions for the innovation, competing priorities, and innovation overload which may lead to burnout. Finally, a critical factor in adoption is the costs of implementation and whether these can be recovered by insurance payment for services provided or need to be considered as up-front costs to achieve downstream savings (e.g., by reduced hospitalization). If the latter, then the degree of financial alignment of components of the health system (hospital, medical group) and timeframe for cost recovery are important. Of note, despite the addition of dementia as a complexity modifier in value-based care, we are not aware of any accountable care or managed care organization that has specifically identified members with dementia as targets for a comprehensive dementia care program.
Innovations in dementia care have had obstacles to dissemination despite effectiveness in randomized clinical trials.12,13 In the 15 years since these trials were published, there has been little uptake of these interventions at other sites. Most recently, a National Academies of Science, Engineering, and Medicine report concluded that there is sufficient evidence to begin broad dissemination of Resources for Enhancing Alzheimer’s Caregiver Health (REACH) II and collaborative care models.14
In disseminating the UCLA Alzheimer’s and Dementia Care Program, we attempted to overcome many of these barriers through strategies such as non-traditional communication methods (e.g., enlisting respected professional societies and organizations), working with adopting sites to customize the program to fit the local environment (e.g., modifying the electronic health record support to different platforms, building ADC functions into existing responsibilities of advanced practice providers already working at the institution, adding complimentary roles for social workers and registered nurses), providing extensive technical assistance through the period of initial adoption and program setup, and assisting adopting sites to create financial plans that justify investment in the program. The approach also draws from principles of Agile implementation,15 including teams of cross-functional developers, project managers, project facilitators, and stakeholders and flexibility in implementation to achieve the broader goal of improved dementia care. Although only a minority of health systems expressing interest have yet implemented the ADC Program, these early adopters are important in the diffusion curve.10
As we move into the next phase of dissemination, the ADC program plans to implement two major facilitators of spread. First, we plan to create an ADC Dissemination Center to handle inquiries about the program, help sites through pre-adoption tasks, apply fidelity metrics, advise on billing for clinical services, and train professional and non-licensed staff. This training is particularly important because many newly graduated advance practice providers have had little training in geriatrics, much less in dementia care. This Center will also accommodate the extensive need for support at some adopting sites. To support and sustain the Dissemination Center, future adopting sites will be assessed a fee to cover the costs of personnel time and materials. We also will train two types of trainers for new DCSs. The first type will be a DCS from an adopting site who can train additional DCSs to spread the program within the site. The second type will be DCSs who will help the Dissemination Center increase its capacity to expand the number of new adopting sites.
Second, we plan to establish a National Dementia Care Learning Collaborative, envisioned as an ongoing community of practice including both sites implementing the model and sites considering adoption. The Collaborative will allow participants to create solutions to implementation barriers in their local environments, grow new professional networks, and share best practices. Similar strategies have been successful in spreading innovations such as the Nurses Improving Care of the Hospitalized Elderly (NICHE), Hospital at Home, and Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) programs.
Finally, to extend spread beyond early adopters, health systems will need to be able to provide clinical services of the program without taking a financial loss. Current fee-for-service and alternative payment model systems do not reimburse adequately for the services required to implement and sustain comprehensive dementia care programs such as the ADC Program. Although the Affordable Care Act granted the Center for Medicare and Medicaid (CMS) the authority to implement payment for successful programs supported by Health Care Innovation Awards, including the ADC Program, few successful clinical programs have led to new payment for services. Thus, new payment models will be needed if providing high quality dementia care through innovations such as the ADC Program are to be implemented widely. Pending legislation (the Comprehensive Care for Alzheimer’s Act)16, if passed and signed into law, as well as efforts within CMMI may make a compelling case for the relative advantage of the ADC Program and other collaborative care models, and dramatically accelerate dissemination.
With the large and growing numbers of persons living with dementia17 and the small number of health systems that have formal, effective comprehensive dementia care programs, scaling up dissemination of collaborative care models, such as the Alzheimer’s and Dementia Care (ADC) Program, is critically important and urgent.
Supplementary Material
Supplementary Description S1. One-page summary describing what the implementation team could provide
Supplementary Figure S1. Readiness Assessment Instrument
Supplementary Table S1. Table of adopting sites and timelines for adoption
Key points:
Although there is substantial interest in adopting successful dementia care programs, in the current health care environment, only a minority of health systems expressing interest have been able to commit to adoption.
Models of dementia care need to be flexible enough to allow adopting health care systems to adapt them to fit their local cultures and workflow as well as clinical training in dementia and the model of care.
Financial reimbursement for comprehensive dementia clinical services beyond traditional clinical encounters remains an important barrier to broad dissemination.
Why Does this Paper Matter?
With the large and increasing numbers of older persons living with dementia, health systems will need to implement dementia care programs that lead to better care, improved patient and caregiver outcomes, and lower costs.
Support:
Research reported in this publication was supported by The John A. Hartford Foundation, the National Institute on Aging of the National Institutes of Health under Award Numbers R21AG054681, and The Commonwealth Fund.
Sponsor’s Role:
The funders had no role in the study design, data collection, analysis and interpretation of data, writing of the report, or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: Disclaimers: The views expressed in this article are the authors’ and do not necessarily represent the views of The John A. Hartford Foundation, the National Institutes of Health, or The Commonwealth Fund, its directors, officers, or staff.
Conflict of Interest: None
REFERENCES
- 1.Gitlin LN, Marx K, Stanley IH, Hodgson N. Translating Evidence-Based Dementia Caregiving Interventions into Practice: State-of-the-Science and Next Steps. Gerontologist. 2015. Apr;55(2):210–26. doi: 10.1093/geront/gnu123. Epub 2015 Feb 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanney SR, Castle-Clarke S, Grant J, et al. How long does biomedical research take? Studying the time taken between biomedical and health research and its translation into products, policy, and practice. Health Res Policy Syst. 2015;13:1. Published 2015 Jan 1. doi: 10.1186/1478-4505-13-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reuben DB, Evertson LC, Wenger NS, et al. The University of California at Los Angeles Alzheimer’s and Dementia Care program for comprehensive, coordinated, patient-centered care: preliminary data. J Am Geriatr Soc. 2013. Dec;61(12):2214–2218. doi: 10.1111/jgs.12562. Epub 2013 Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berwick DM, Nolan TW, Whittington J. The Triple Aim: Care, Health, And Cost Health Affairs, 27, no.3 (2008):759–769 [DOI] [PubMed] [Google Scholar]
- 5.Jennings LA, Tan Z, Wenger NS, et al. Quality of Care Provided by a Comprehensive Dementia Care Comanagement Program. J Am Geriatr Soc. 2016. Aug;64(8):1724–30. doi: 10.1111/jgs.14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reuben DB, Tan ZS, Romero T, et al. Patient and Caregiver Benefit From a Comprehensive Dementia Care Program: 1-Year Results From the UCLA Alzheimer’s and Dementia Care Program. J Am Geriatr Soc. 2019. Nov;67(11):2267–2273. doi: 10.1111/jgs.16085. Epub 2019 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jennings LA, Laffan AM, Schlissel AC, et al. Health Care Utilization and Cost Outcomes of a Comprehensive Dementia Care Program for Medicare Beneficiaries. JAMA Intern Med. 2018. Dec 21. doi: 10.1001/jamainternmed.2018.5579. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennings LA, Hollands S, Keeler E, Wenger NS, Reuben DB. The Effects of Dementia Care Co-Management on Acute Care, Hospice, and Long-Term Care Utilization. J Am Geriatr Soc. 2020. Nov;68(11):2500–2507. doi: 10.1111/jgs.16667. Epub 2020 Jun 23. [DOI] [PubMed] [Google Scholar]
- 9.Reuben DB, Gill TM, Stevens A, et al. D-CARE: The Dementia Care Study: Design of a Pragmatic Trial of the Effectiveness and Cost Effectiveness of Health System-Based Versus Community-Based Dementia Care Versus Usual Dementia Care. J Am Geriatr Soc. 2020. Nov;68(11):2492–2499. doi: 10.1111/jgs.16862. Epub 2020 Oct 6. Erratum in: J Am Geriatr Soc. 2021 Apr 30; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers, Everett (16 August 2003). Diffusion of Innovations, 5th Edition. Simon and Schuster. ISBN978-0-7432-5823-4. [Google Scholar]
- 11.Reckrey JM, Gazarian P, Reuben DB, et al. Barriers to implementation of STRIDE, a national study to prevent fall-related injuries. J Am Geriatr Soc. 2021. Feb 13. doi: 10.1111/jgs.17056. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006. May 10;295(18):2148–57. [DOI] [PubMed] [Google Scholar]
- 13.Vickrey BG, Mittman BS, Connor KI, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med. 2006. Nov 21;145(10):713–26. [DOI] [PubMed] [Google Scholar]
- 14.National Academies of Sciences, Engineering, and Medicine 2021. Meeting the Challenge of Caring for Persons Living with Dementia and Their Care Partners and Caregivers: A Way Forward. Washington, DC: The National Academies Press. 10.17226/26026. [DOI] [PubMed] [Google Scholar]
- 15.Boustani M, Alder CA, Solid CA. Agile Implementation: A Blueprint for Implementing Evidence-Based Healthcare Solutions. J Am Geriatr Soc. 2018;66(7):1372–1376. doi: 10.1111/jgs.15283 [DOI] [PubMed] [Google Scholar]
- 16. [act accessed 10/2/2021]. https://alzimpact.org/priorities/comprehensive
- 17.Alzheimer’s Association. 2021 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2021;17(3) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Description S1. One-page summary describing what the implementation team could provide
Supplementary Figure S1. Readiness Assessment Instrument
Supplementary Table S1. Table of adopting sites and timelines for adoption


