FIGURE 1.
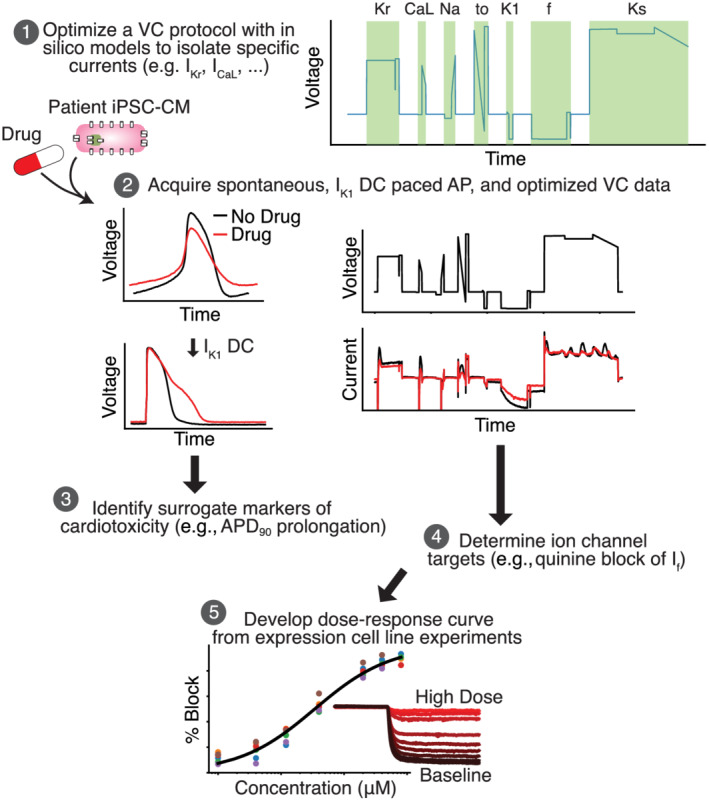
An in silico–in vitro pipeline to determine drug cardiotoxicity risk and mechanism. Step 1, the Kernik–Clancy model with experimental artefacts is used to develop a voltage clamp (VC) protocol that is specifically designed to isolate currents. Step 2, spontaneous, IK1 dynamic clamp and paced action potentials (AP), and optimized VC data are acquired from a patient‐derived induced pluripotent stem cell‐derived cardiomyocyte (iPSC‐CM) before and after drug application. Step 3, the change in IK1 dynamic clamp and paced AP data from pre‐ to post‐drug application is used to identify AP prolongation, a surrogate marker of cardiotoxicity. Step 4, changes in VC data are used to determine the ion channels targeted by a drug. Step 5, after identifying the ion channel targeted by a drug, a dose–response curve is developed for each of these ion channels using expression line cells.
