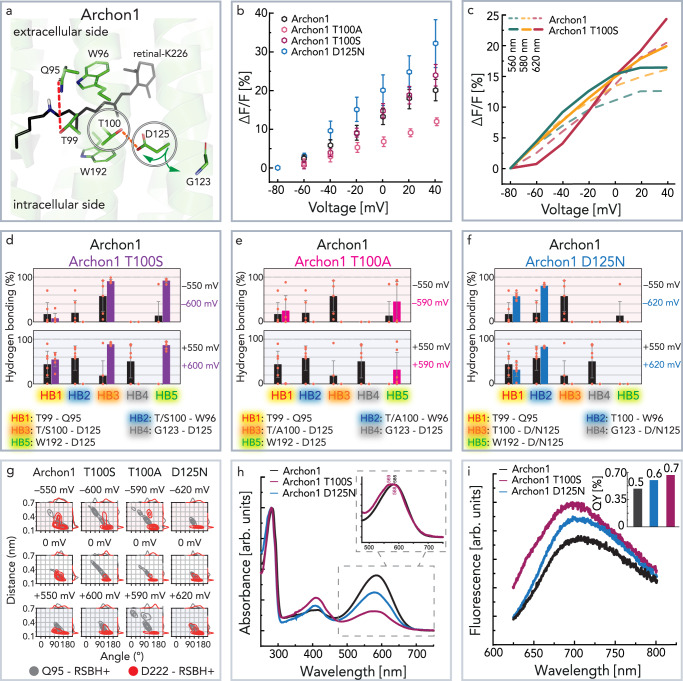
Fig. 4. Archon1-T100S/A and Archon1-D125N.
a Retinal binding pocket highlighting mutated residues—T100 and D125. b Voltage-dependent increase in fluorescence intensity of Archon1, Archon1-T100A, Archon1-T100S, Archon1-D125N at continuous 620 nm excitation RT, pH 7.2 (Archon n = 14; Archon1-T100A n = 4, p-value 0.184; Archon1-T100S n = 4, p-value 0.367; Archon1-D125N n = 4, p-value 0.080, the p-values are determined according to Wilcoxon–Mann–Whitney test (two-sided), Supplementary Fig. 22, Supplementary Table 3). c Comparison between Archon1 (dashed lines) and Archon1-T100S (solid lines) voltage regulated fluorescence intensity (ΔF/F) dependent on excitation wavelength −560 nm (green, p-value 0.327), 580 nm (orange, p-value 0.313), 620 nm (red) (n = 4, p-value 0.367, the p-values are determined according to Wilcoxon–Mann–Whitney test (two-sided), Supplementary Fig. 22, Supplementary Table 3), measured at pH 7.2 and RT. d–f Voltage-dependent probability of important hydrogen bonds in T100S (purple), T100A (pink), and D125N (blue) compared to Archon1 (black) using the same nomenclature as in Fig. 3 (the number of simulations for each voltage = 6). Data are presented as mean values ± SD. Each orange circle represents the data derived from the individual MD trajectories. g Distance and angle distributions between the RSBH+ and the two counterions Q95 (gray) and D222 (red) as observed by MD simulations at negative, zero, and positive transmembrane voltages in Archon1, Archon1-T100S, Archon1-T100A, and Archon1-D125N. d–g The MD simulations were conducted at 303 K by fixing the protonation states of titratable sites at neutral pH. h Absorption spectra comparing Archon1, Archon1-T100S, and Archon1-D125N. i Fluorescence emission and QY. Spectra of panels h and i have been recorded at RT, pH 8.0. Source data are provided in the Source Data file (b–i).