Abstract
Background
We estimate effectiveness of 3 versus 2 vaccine doses against SARS-CoV-2 B.1.1.529 Omicron in a mostly infection-naiive but highly vaccinated Australian population.
Methods
Cohort study of adults aged 40+ years resident in Sydney followed from 1 January 2022 for SARS-CoV-2 infection and COVID-19 hospitalisation or death using linked immunisation, disease notification and hospitalisation registers. Adjusted hazard ratios (aHR) and corresponding relative vaccine effectiveness (rVE) were estimated comparing 3 to 2 vaccine dose recipients by time since dose receipt, vaccine brand, and prior infection. Absolute risk reductions and numbers needed to boost by age groups were calculated.
Results
2,053,123 infection-naiive individuals (mean age 59 years) were followed for 327,272 person-years for infection and 224,269 person-years for severe outcomes (hospitalisation/death). There were 175,849 infections and 4113 hospitalisations/deaths. Compared to individuals receiving dose 2 within the last 3 months, rVE in dose 3 recipients was 7% (95% CI 5–9%) against infection and 65% (95%CI 61–69%) against hospitalisation/death. Almost all dose 3 recipients had an mRNA vaccine; there was little difference in dose 3 rVE by primary course vaccine brand (ChAdOx1 versus BNT162b2). Over the 6-week follow-up, we estimated one hospitalisation/death was avoided for every 192 adults aged ≥70 years boosted with dose 3 in the infection-naiive cohort. The aHR for hospitalisation/death from Omicron was 0.12 (95 %CI 0.07–0.23) for 2-dose recipients with a prior Delta infection compared with 2-dose recipients with no prior infection.
Conclusions
Receipt of a third COVID-19 vaccine dose in adults aged 40 years and above significantly reduced hospitalisations and deaths from SARS-CoV-2 Omicron infections in a primarily infection-naiive population.
1. Introduction
The SARS-CoV-2 Omicron variant of concern (VOC) was first detected on 9 November 2021 in South Africa and rapidly spread worldwide.[1] Significant mutations on the spike protein have contributed to the variant’s ability to escape both infection- and vaccine-induced immunity.[2], [3] The first known introduction of Omicron into Australia was on 23 November 2021.[4] The Australian population had been subject to strict border controls limiting movement and requiring quarantine on entry until 1 November 2021. As a result, Australia had experienced a very low SARS-CoV-2 infection rate with ∼200,000 cumulative cases[5] among more than 25 million people since the pandemic began in 2020. While at the time of Omicron introduction the Australian population was mostly infection naiive,[6] very high rates of vaccination had been achieved, with 85.5% of those aged 16+ years having received 2 doses of COVID-19 vaccines by 23 November 2021.[7] A rapid rise in COVID-19 cases occurred in Australia during December 2021 suggesting reduced immune protection and prompting faster roll-out of a third vaccine dose to all individuals aged 16+ years with a reduction in the recommended interval between dose 2 and dose 3 to 3 months.[8] Despite this, by mid-January, Australia was reporting much higher daily COVID-19 case rates than at any time previously.[9].
Almost all data on the effectiveness of COVID-19 vaccines has derived from settings where there had already been substantial community transmission of SARS-CoV-2.[10] As infection itself has been shown to induce significant protective immunity against subsequent disease,[11] it is possible that vaccine effectiveness seen in these settings may not be replicated in populations with low infection rates. Prior to introduction of the Omicron VOC, extensive control strategies and very high testing rates meant there was high ascertainment of SARS-CoV-2 infections in the Australian community as demonstrated through low test positivity and serosurveys.[6], [12] We therefore sought to estimate and compare the relative vaccine effectiveness (rVE) of 3 compared to 2 COVID-19 vaccine doses and the impact of prior infection on VE in relation to infection and severe disease (hospitalisation or death) from the Omicron VOC in a mostly infection naiive population.
2. Methods
2.1. Study design and data sources
We conducted a cohort study among individuals 40 years and older residing in the Greater Sydney Area (Australia’s largest city within its most populous state, New South Wales [NSW]). The population was defined using the Australian Immunisation Register (AIR) which is a national Register including all people in Australia registered with Australia’s national health insurance scheme (Medicare) as well as individuals not registered but who receive a COVID-19 vaccine. The AIR includes demographic information on individuals and vaccine details (brand, date of administration, dose number). Reporting of COVID-19 vaccination to the AIR was made mandatory prior to implementation of the COVID-19 vaccine program in March 2021.
The cohort was probabilistically linked (using name, date of birth, residential address and sex) to notifications of SARS-CoV-2 infections, and hospitalisation and death registries. Notification of SARS-CoV-2 infection is mandatory with date and method of diagnosis recorded in the Notifiable Conditions Information Management System (NCIMS). For this study, diagnoses made by polymerase chain reaction (PCR) tests or rapid antigen tests (RAT) were included as both forms of test were required to be notified. The NCIMS also includes information on whether an individual was known to have died with COVID-19 based on information reviewed by the local public health unit. Hospitalisation data covered all NSW public hospitals (providing virtually all care for COVID-19 patients in NSW) and included episodes of care where an individual was hospitalised at the time of the data extract. Where available, up to 51 assigned diagnosis codes (coded using the International Classification of Diseases version 10 [ICD-10] coding system) and admission and discharge dates were included in hospitalisation data. Death registrations included all registered deaths in NSW and the date of death.
2.2. Study definitions
Two outcomes were examined: 1) SARS-CoV-2 infection based on case notification in the NCIMS and, 2) severe COVID-19 defined as either a hospitalisation or death related to SARS-CoV-2 infection. Individuals were classified as having a COVID-19 hospitalisation if they had a NCIMS case notification and were admitted within 48 hours prior to and up to 14 days after the date of diagnosis and if the patient was admitted to hospital ward types typically used for treatment of COVID-19 cases (including intensive care units; see Appendix). A COVID-19 death was ascertained from NCIMS reports or if an individual with a COVID-19 notification had a registered death in the 48 hours prior to and up to 30 days post infection. Vaccination data was obtained from each individual’s AIR record.
2.3. Statistical analysis
For the main analyses we estimated the rVE of 3 compared to 2 doses of vaccine in the infection-naiive population. The study period started from 1 January 2022 as this corresponded to a period when greater than 90% of all isolates sequenced in Australia were estimated to be the Omicron VOC B.1.1.529.[13] For these analyses we excluded individuals who had a COVID-19 case notification or a death record prior to 1 January 2022. We also excluded unvaccinated individuals as they may differ from vaccinated people in many behavioural characteristics (including health-seeking behaviour) and we could not be certain they were resident in Australia during the study period.
We used Cox-regression with vaccination status as a time-varying covariate, and conducted separate analyses for each outcome. Analysis time commenced on 1 January 2022 and ended at either the outcome of interest, death, receipt of a 4th vaccine dose or the last date of complete records for each endpoint, whichever came first. For SARS-CoV-2 case notifications we had complete records to 4 March 2022 and for severe disease (hospitalisations/death) to 10 February 2022.
To estimate rVE, the reference group was individuals in the 8–89 days after receipt of 2-doses of vaccine. We used this group as by January 2022 the recommended interval between receipt of dose 2 and dose 3 was 3 months.[8] We calculated adjusted hazard ratios (aHR) in relation to this group according to different dose numbers and time since receipt, specifically: 90–179 days after 2 doses; 180+ days after 2 doses; 0–7 days after 3 doses; 8+ days after 3 doses. Analyses were adjusted for age (in 2-year increments), sex, socioeconomic status (deciles based on an Australian index using residence and census data[14]), and number of comorbidities (based on ICD-10 coded hospitalisations with specified medical conditions in the 2 years prior to the analysis start date – see appendix for details). Relative VE was calculated as (1 – aHR) * 100 %.
We examined rVE overall and according to primary course brand (ChAdOx1; Astrazeneca, BNT162b2; Pfizer). For brand-specific analyses those who received 2 doses of the Pfizer BNT162b2 vaccine within 8–89 days were the reference group. As the Pfizer BNT162b2 vaccine was preferentially given to certain populations (initially healthcare and border workers, and residents of aged care facilities, and then later those aged <50 years expanding to those <60 years[8]) we restricted brand-specific comparative analyses to 50–69 year olds in which uptake of the two vaccine brands was approximately equal. We also conducted subgroup analyses by age (<65 and ≥65 years), presence of comorbidities (yes, no) and excluding individuals with a record of an immunocompromising condition (as a 3rd vaccine dose was recommended for this group prior to the general population[8]).
Additional analyses included individuals who had a prior SARS-CoV-2 case notification from 16 June to 31 December 2021; which corresponded to the Delta outbreak period in Sydney[15]. We compared risks of infection and severe disease from 1 January 2022 among those who received 2 doses according to whether they had a prior infection. At least 91 days had to have elapsed between notified positive test results for a subsequent infection to be included as an outcome in these analyses. Adjusted absolute rates of severe disease according to vaccination status and age were estimated using rates of severe disease in the reference population (from quasi-Poisson regression) and multiplying these rates by the aHRs. Absolute risk reductions and numbers needed to treat were approximated using Austin and Altman methods.[16].
This study was conducted as part of NSW public health COVID-19 management and an ethics committee determined there were no ethical risks requiring submission to an HREC.
3. Results
After exclusions, the main study cohort included 2,056,123 individuals. Table 1 shows characteristics of the cohort, including vaccination status at 1 January 2022. The average age was 59.0 years (standard deviation [SD] 13.3 years) and 51.3% were women. Reflecting the cohort age and residence in Sydney, compared to the whole Australian population, 75.6% were classified above the median Australian socioeconomic index; 11.0% had a least one comorbidity. At January 1, 76.7% had received 2 doses of a COVID-19 vaccine, 22.3% 3 doses and 1.0% one dose; as described earlier, those with no reported vaccine doses were excluded from analyses. Individuals who had 3 doses of vaccine were slightly older than 2 dose recipients, more likely to be women, have higher socioeconomic status and have a comorbidity. For those who received 2 doses, 52.4% received BNT162b2 (mean interval between dose 1 and 2 was 3.6 weeks [SD 1.5; median 3]) and 46.8% ChAdOx1 (mean interval between dose 1 and 2 was 8.7 weeks [SD 3.0; median 8.3]). For dose 3, 85.4% received BNT162b2 and 13.7% received mRNA-1273 (Moderna).
Table 1.
Characteristics of cohort overall and according to vaccine doses received at beginning of study period during Omicron outbreak (1 Jan 2022).
| 1 dose | 2 doses | 3 doses | Total | |||||
|---|---|---|---|---|---|---|---|---|
| N | 19,849 | 1,575,405 | 457,869 | 2,053,123 | ||||
| Mean age (SD) | 59.5 (14.6) | 58.3 (13.0) | 61.4 (13.9) | 59.0 (13.3) | ||||
| Age group (years) | ||||||||
| 40–49 | 31.4 % | 6,228 | 30.8 % | 484,912 | 25.6 % | 117,306 | 29.6 % | 608,446 |
| 50–59 | 24.5 % | 4,855 | 27.1 % | 426,748 | 23.0 % | 105,182 | 26.1 % | 563,785 |
| 60–69 | 19.9 % | 3,950 | 21.6 % | 340,206 | 20.4 % | 93,342 | 21.3 % | 437,498 |
| 70–79 | 12.5 % | 2,479 | 13.1 % | 207,029 | 19.5 % | 89,126 | 14.6 % | 298,634 |
| 80–89 | 8.1 % | 1,605 | 6.0 % | 95,009 | 8.9 % | 40,767 | 6.7 % | 137,381 |
| 90+ | 3.7 % | 732 | 1.4 % | 21,501 | 2.6 % | 12,146 | 1.7 % | 34,379 |
| % Women (N) | 49.5 % | 9,817 | 51.0 % | 803,835 | 52.5 % | 240,164 | 51.3 % | 1,053,816 |
| Proportion above median Australian socioeconomic index % (N) | 66.5 % | 13,202 | 73.6 % | 1,159,061 | 82.8 % | 379,041 | 75.6 % | 1,551,304 |
| % At least one comorbidity (N) | 12.0 % | 2,390 | 10.3 % | 161,686 | 13.4 % | 61,268 | 11.0 % | 225,344 |
| COVID-19 vaccine brand | ||||||||
| Astra Zeneca (ChAdOx1) % (N) | 32.3 % | 6,415 | 46.8 % | 737,301 | 0.9 % | 4,241 | ||
| Moderna (mRNA-1273) % (N) | 4.8 % | 953 | 0.8 % | 12,033 | 13.7 % | 62,655 | ||
| Pfizer (BNT162b2) % (N) | 62.2 % | 12,349 | 52.4 % | 825,301 | 85.4 % | 390,865 | ||
| Other % (N) | 0.7 % | 132 | 0.1 % | 770 | 0.0 % | 108 | ||
| Mean weeks since receipt of last dose (SD) | 13.4 (10.0) | 16.8 (5.0) | 2.4 (2.7) | |||||
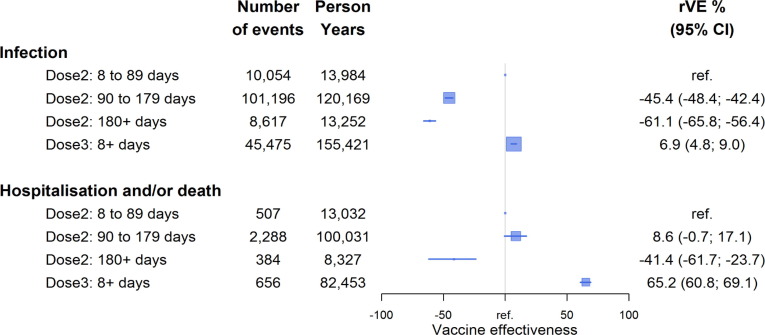
Table 2 shows case numbers, time at risk, and hazard ratios for infection and severe disease (hospitalisation/death) by vaccine dose and time since receipt. Fig. 1 shows rVE compared to 8–89 days after 2 doses for all vaccine brands combined. There were 175,849 SARS-CoV-2 infections reported over 327,272 person-years (of which 16% were diagnosed only with a RAT). For severe disease there were 4113 events (3812 hospitalisations and 670 deaths; 301 deaths were not hospitalised) over 224,269 person-years. Compared to those who received dose 2 in the first 8–89 days, risk of infection increased in the 3–6 months and ≥ 6 months after dose 2 receipt (aHR 1.45, 95%CI 1.42–1.48 and aHR 1.61, 95%CI 1.56–1.66 respectively) but in the ≥ 8 day period after dose 3 receipt (mean time since receipt 7.0 weeks), risk was modestly lower (aHR 0.93, 95%CI 0.91–0.95). While rVE against infection waned substantially from 3 months after receipt of dose 2, rVE against severe infection was better preserved until 6 months. The rVE of dose 3 (given on average 4.8 weeks prior [time period differs due to different length of follow-up for outcomes]) against severe disease was substantially better than that against infection: rVE 65% (95%CI 61–69%) compared to dose 2 in the first 8–89 days. When examined in subgroups, rVE of dose 3 versus 2 against hospitalisation or death was slightly lower among 40–64 year olds compared to those ≥65 years (47% [95%CI 34–57%] vs 70% [95%CI 65–74 %]) and in individuals with comorbidities (60% [95%CI 53–66]) (Appendix B).
Table 2.
Number, person-time and hazard ratios for SARS-CoV-2 Omicron infection and severe disease (hospitalisation/death) according to vaccination status.
| SARS-CoV-2 infections from 1 Jan to 4 Mar 2022 |
SARS-CoV-2 Hospitalisation or deaths from 1 Jan to 10 Feb 2022 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (events) |
Person Years | Adjusted Hazard Ratio* | 95 % LCI | 95 % UCI | N (events) |
Person Years | Adjusted Hazard Ratio* | 95 % LCI | 95 % UCI | ||
| Dose 1 | 1,316 | 2,512 | 1.014 | 0.958 | 1.074 | 88 | 1,821 | 1.330 | 1.060 | 1.669 | |
| Dose 2–0 to 7 days | 62 | 143 | 0.750 | 0.584 | 0.963 | 6 | 123 | 0.978 | 0.437 | 2.187 | |
| Dose 2–8 to 89 days | 10,054 | 13,984 | ref | – | – | 507 | 13,032 | ref | – | – | |
| Dose 2–90 to 179 days | 101,196 | 120,169 | 1.454 | 1.424 | 1.484 | 2,288 | 100,031 | 0.914 | 0.829 | 1.007 | |
| Dose 2 - ≥180 days | 8,617 | 13,252 | 1.611 | 1.564 | 1.658 | 384 | 8,327 | 1.414 | 1.237 | 1.617 | |
| Dose 3–0 to 7 days | 9,129 | 21,792 | 0.824 | 0.801 | 0.848 | 184 | 18,483 | 0.379 | 0.319 | 0.449 | |
| Dose 3 - ≥ 8 days | 45,475 | 155,421 | 0.931 | 0.910 | 0.952 | 656 | 82,453 | 0.348 | 0.309 | 0.392 | |
*Adjusted for age, sex, socioeconomic status, co-morbidity (see methods for definitions).
Fig. 1.
Vaccine effectiveness relative to within 8–89 days of receipt of 2-doses of COVID-19 vaccine against SARS-CoV-2 Omicron infection and severe disease (hospitalisation/death). Squares represent the point estimate of vaccine effectiveness and their size is inversely proportional to the amount of data; lines represent the 95% confidence intervals with arrows indicating if the limit of the confidence interval goes beyond the scale shown. The reference category has no confidence interval. Relative VE estimate adjusted for age, sex, socioeconomic status, co-morbidity (see methods for definitions).
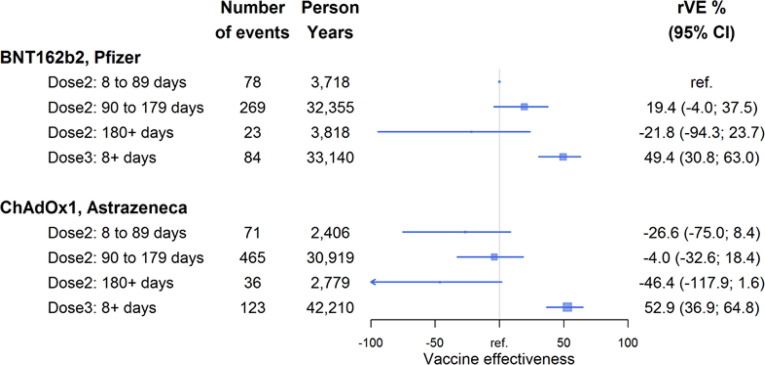
Among 50–69 year olds, 47.2% had received BNT162b2 and 52.0% had ChAdOx1 vaccine for their primary course by 1 Jan 2022 (there were almost no mixed brand recipients). Fig. 2 shows rVE by primary vaccine brand against severe disease restricted to this age group with the reference group receipt of 2 doses of the BNT162b2 vaccine in the first 8–89 days. There was evidence of waning effectiveness of 2 doses by 6 months after receipt against severe disease and boosted protection with dose 3 but no substantial difference in vaccine effectiveness by the primary course brand.
Fig. 2.
Vaccine effectiveness relative to within 8–89 days of receipt of 2-doses of BNT162b2 Pfizer vaccine (reference) against SARS-CoV-2 Omicron severe disease (hospitalisation/death) by vaccine brand of primary course in 50–69 year olds. Squares represent the point estimate of vaccine effectiveness and their size is inversely proportional to the amount of data; lines represent the 95% confidence intervals with arrows indicating if the limit of the confidence interval goes beyond the scale shown. The reference category has no confidence interval. Relative VE estimate adjusted for age, sex, socioeconomic status, co-morbidity (see methods for definitions).
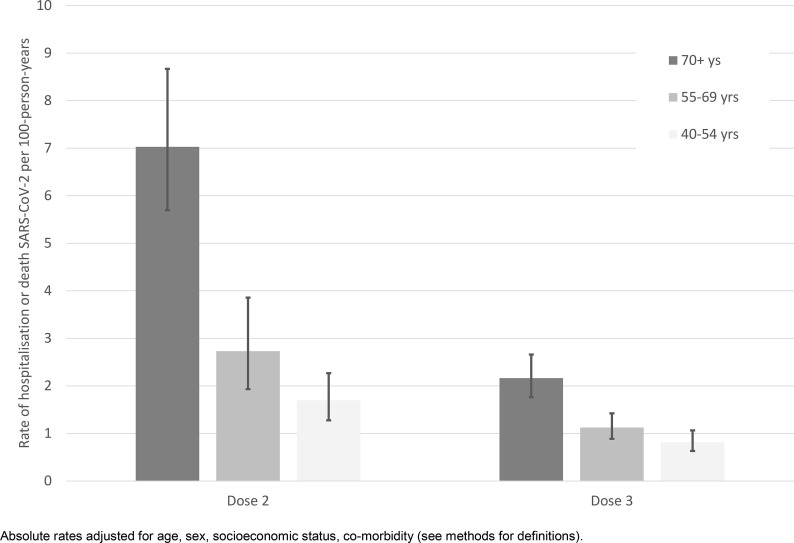
Fig. 3 shows the absolute rates of hospitalisation or death from SARS-CoV-2 according to both age and vaccination status (2 doses within 8–89 days vs 3 doses given on average 34 days ago). The absolute risk reduction in the 6 week period from 1 January 2022 for an individual aged ≥70 after vaccine dose 3 compared to up to 3 months post-dose 2 was approximately 5.2/1000; therefore for every 192 people who received a third vaccine dose, one hospitalisation or death was avoided in this period. For individuals aged 55–69 and 40–54 years at least one hospitalisation or death was avoided for every 1007 and 2314 people who received dose 3, respectively.
Fig. 3.
Adjusted absolute rates of SARS-CoV-2 Omicron severe disease (hospitalisation/death) by age group and within the first 3 months of receipt of vaccine dose 2 or 3. Absolute rates adjusted for age, sex, socioeconomic status, co-morbidity (see methods for definitions).
An additional 43,288 individuals aged 40+ years resident in Greater Sydney in the AIR database with at least one vaccine record had a SARS-CoV-2 case notification before 1 January 2022. When this group with a history of prior infection was included in the main cohort there were an additional 352 SARS-CoV-2 infections and 16 COVID-19 hospitalisations/deaths in the study period. Among those with a prior infection who received 2 doses, the aHR for both infection and severe COVID-19 were substantially lower than in infection-naiive individuals who had received 2 doses of vaccine (aHR 0.06; 95%CI 0.055–0.07 and 0.12; 95%CI 0.07–0.23 respectively; Table 3 ).
Table 3.
Number, person-time and hazard ratios for SARS-CoV-2 Omicron infection and severe disease (hospitalisation/death) in dose 2 recipients according to prior infection status.
| SARS-CoV-2 infections from 1 Jan 2022 to 4 Mar 2022 |
SARS-CoV-2 Hospitalisation or deaths from 1 Jan 2022 to 10 Feb 2022 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean time (weeks) since vaccine receipt (SD) | N | Person Years | Adjusted Hazard Ratio* | 95 % LCI | 95 % UCI | Mean time (weeks) since vaccine receipt (SD) | N | Person Years | Adjusted Hazard Ratio* | 95 % LCI | 95 % UCI | |
| Dose 2 | ||||||||||||
| Infection naiive | 22.0 (4.6) | 119,929 | 147,548 | ref | 21.2 (4.5) | 3,185 | 121,513 | ref | ||||
| Prior infection | 23.3 (6.5) | 251 | 4,816 | 0.062 | 0.055 | 0.071 | 21.5 (6.4) | 10 | 3,458 | 0.122 | 0.066 | 0.227 |
Dose 2 includes all individuals receiving that dose regardless of the time since receipt.
*Adjusted for age, sex, socioeconomic status, co-morbidity (see methods for definitions). For someone with a prior infection to be classified as having a new infection a period of at least 91 days had to separate the infection notifications.
4. Discussion
In this highly vaccinated but predominantly infection-naiive older Australian population we found recent receipt of a third dose of an mRNA COVID-19 vaccine improved protection against severe disease from the Omicron BA.1 VOC by about 65% compared to recent receipt of a second COVID-19 vaccine dose. The absolute risk reduction in the first 4–5 weeks following a third dose was greatest in people aged 70+ years with an estimated 1 hospitalisation or death from the Omicron VOC avoided for every 192 people boosted. We did not find significant differences in protection against Omicron infection outcomes when comparing 2 dose primary vaccination with ChAdOx1 vaccine (with a median 8 weeks between doses) and BNT162b2 vaccine (3-week dose interval), noting most third (booster) doses were mRNA vaccines. We also estimated in people who received 2 doses of vaccine, those with infection during the Delta outbreak period (in the 6 months earlier) who survived until 1 January 2022 had a lower risk of severe Omicron disease than infection-naiive individuals who had received 2 doses of vaccine.
In this study we calculated relative vaccine effectiveness because such a high proportion of the Australian population was vaccinated with at least 2 doses. Vaccine effectiveness estimates using the unvaccinated population as a reference would be easily biased by small misclassifications of vaccine status or outcome; confounding by behavioural differences may also occur and this is difficult to control. Reporting of relative vaccine effectiveness has been used in other settings to compare the incremental impact of additional COVID-19 vaccine doses.[17] Overall, our findings are comparable to other studies reporting either relative or absolute vaccine effectiveness, which show rapid waning of 2-dose vaccine effectiveness against Omicron infection, and short-term restoration of protection against infection with a booster dose.[18], [19], [20], [21] Also similar to these other studies we found protection with 2 vaccine doses against severe disease following Omicron was more durable and that the effectiveness significantly improved with dose 3.[19], [22].
Our study is somewhat unique in that the vast majority of the population had never been infected with SARS-CoV-2 due to Australia’s strict border controls and absent to low community transmission rates up until late 2021.[12] This enabled us to assess differences in protection from Omicron in those previously infected and uninfected with the Delta variant. We found that among 2-dose vaccine recipients, recent prior Delta infection was associated with higher protection against severe disease from Omicron, but small case numbers precluded equivalent comparisons among individuals who received a third vaccine dose. Our findings regarding hybrid immunity are consistent with some other studies of vaccine effectiveness [23], [24], [25] and immune response to vaccination and infection[26], [27] which suggest each vaccine dose and/or infection with SARS-CoV-2 can provide incremental improvements in protection against subsequent severe disease over time. However as indicated by the World Health Organization,[28] in order to optimise future use of vaccines, more data is required to better understand how differences in the variant that individuals were infected with, and the number, timing and type of vaccine dose, may affect subsequent infection risk.
Rates of severe disease defined as hospitalisation or death from COVID-19 were highest in the oldest population (≥70 years) however, during this peak period of Omicron infection in Australia, we estimated this reduced by at least 5.2/1000 with receipt of dose 3. This demonstrates in this infection naiive population, the substantial benefits of booster dose vaccination against the Omicron variant, even with high rates of community transmission. The absolute risk reductions were smaller for adults aged 40–69 years however, at the population level, a third dose still contributed substantially to overall reductions in hospitalisations.
Our study strengths include the large population, use of a national vaccine register to ascertain vaccination status and a cohort analysis which allowed estimation of rates of infection and severe disease. Limitations are similar to other observational studies of vaccine effectiveness. This includes that ascertainment of infections was restricted to those tested and could be biased to those more likely to seek medical care and be vaccinated. Also ascertainment of severe disease using hospitalisations as a proxy may be biased if diagnosis of infection is incidental to the hospital admission rather than a contributory factor.[29] However, we were able to use ward admission types to assist in classifying hospitalisations related to Omicron, a method consistent with studies of severe disease conducted early in the pandemic[30] and showed that our method found a large proportion of individuals where the hospitalisation was likely due to COVID-19 (see Appendix A). Finally ascertainment of infections changed during the course of this study.[31] Prior to 5 January 2022 all individuals testing positive with a RAT were required to have this confirmed by PCR whilst after this date this requirement was removed. Whilst reporting of positive RATs was also mandated, as RATs require individuals to self-notify this is likely to have led to underascertainment of SARS-CoV-2 infections and potential for bias if testing and reporting differ by vaccination status. Hence our study focus was primarily on COVID-19 hospitalisations.
In summary, introduction of the Omicron VOC into this mostly infection-naiive but high 2-dose vaccinated Australian population led to very high rates of infection.[13] Similar to other international studies, this first contemporaneous comparison of booster and brand vaccine effectiveness within the Australian older adult population against Omicron infection following either 2 doses of the Astrazeneca ChAdOx1 or Pfizer BNT162b2 vaccines showed waning of protection in the months after receipt of the second dose that was restored with a third dose. Importantly, protection against severe disease from Omicron was observed to decline more slowly after dose 2 and was significantly enhanced with a third mRNA COVID-19 vaccine dose, affirming the clear protective benefit of booster vaccination in this adult population. Confirmed prior SARS-CoV-2 infection with the Delta variant together with 2 vaccine doses conferred significantly greater protection against subsequent infection and severe disease among survivors than 2 vaccine doses without prior infection. Continued assessments of vaccine effectiveness are needed in different settings around the world to take account of unique population characteristics, vaccine schedules and doses, as well as evolving population immunity from natural infection with different SARS-CoV-2 variants.
Funding: NSW Ministry of Health; HG was funded through the APPRISE CRE Fellowship (NHMRC Grant No: APP1116530).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A.
-
1.
Identification of hospitalisations related to COVID-19.
A person was initially classified as hospitalised for COVID-19 if they had a linked hospital record from the NSW Admitted Patient Data Collection (APDC) and the admission date was within −2 to +14 days of their earliest COVID-19 diagnosis date. Admissions were then excluded if the hospital admission was in a ward type classified as for: obstetrics and gynaecology care, emergency department only, dialysis, rehabilitation, psychiatric care, day-only wards (surgery and medical), spinal or burns, outpatient care (hospital in the home).
Of those classified as hospitalised 56.5% had diagnostic coding reported at the time of data extract. Of those with diagnostic coding 90% had a code indicating COVID-19 or a coronavirus infection (ICD-10 B34.2/ B97.2/ U07.1). Of those with diagnostic coding 37% had a primary diagnostic code for a respiratory system disease (ICD-10J-codes) and 32% had J12.8 or J12.9 other/unspecified viral pneumonia; 24% had codes compatible with symptoms of COVID-19.
-
2.
Identification of comorbidities
To determine comorbidities, all individuals eligible for inclusion in analyses were linked to hospital records from the NSW Admitted Patient Data Collection (APDC) where there was an admission in the 2 years prior to 1 Jan 2022 with any of the ICD-10 diagnostic codes listed below in any field in the hospital record. Individuals were grouped according to the condition and then classified based on the number of comorbidities identified from the linked hospitalisation records.
Respiratory J40-J47; J81; J84; E84.
Diabetes: E10-E14.
Anaemia/splenic issues: D50-D59; D60-D63; D73.
Downs syndrome: Q90.
Cancers C00-C97 excl C44.
Kidney diseases: N18-N19; N00-N16; N25-N29.
Immunocompromise: D80-D89; B24.
Dementia: F00-F03; G30.
Cardiac disease incl hypertension and arrhythmia: I20-I28; I05-I10; I47-I50.
Stroke/TIA: G45; H34; I60-I69.
Liver: K71-K77.
Obesity: E66.
Rheumatoid arthritis: M05-M06.
Ulcerative colitis/Crohns disease: K50-K51.
Appendix B. Adjusted hazard ratio and vaccine effectiveness of 3 versus 2 doses within the first 3 months of receipt against SARS-CoV-2 Omicron severe disease (hospitalisation/death) by age, comorbidities and excluding immunosuppressed individuals
| Cases/Person-years; dose 2 within 8–89 days | Cases/Person-years; dose 3 >=8 days | Adjusted HR dose 3; >=8 days compared to dose 2 within 8–89 days | 95 %LCI | 95 %UCI | Relative vaccine effectiveness 3 vs 2 doses within 8–89 days of receipt | 95 %LCI | 95 %UCI | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All individuals | 507/13032 | 656/82453 | 0.35 | 0.31 | 0.39 | 65.2 | 60.8 | 69.1 | |||
| By age: | |||||||||||
| 40–64 years | 147/8516 | 210/50343 | 0.53 | 0.43 | 0.66 | 46.9 | 33.9 | 57.3 | |||
| 65 + years | 360/4515 | 446/32110 | 0.30 | 0.26 | 0.35 | 69.8 | 65.2 | 73.9 | |||
| By co-morbidity: | |||||||||||
| None reported | 243/11449 | 296/72263 | 0.30 | 0.25 | 0.36 | 69.7 | 64.0 | 74.6 | |||
| At least one | 264/1582 | 360/10190 | 0.40 | 0.34 | 0.47 | 60.1 | 53.0 | 66.1 | |||
| Excluding immunocompromised: | 484/12976 | 590/82064 | 0.33 | 0.29 | 0.37 | 67.3 | 63.0 | 71.1 |
Data availability
The authors do not have permission to share data.
References
- 1.https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern.
- 2.Collie S., Champion J., Moultrie H., Bekker L.-G., Gray G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N Engl J Med. 2021 doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L., Iketani S., Guo Y., et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 4.NSW Health Media Release New Omicron case confirmed in NSW. Date. December 2021;2:2021. [Google Scholar]
- 5.https://www.health.gov.au/health-alerts/covid-19/case-numbers-and-statistics.
- 6.Vette K, Machalek D, Gidding H, et al. Seroprevalence of SARS-CoV-2-specific antibodies in Australia following the first epidemic wave in 2020: a national survey. The Journal of infectious diseases 2022;In press (accepted Jan 2022).
- 7.Australian Government Operation COVID Shield. COVID-19 vaccine rollout update - 23 November 2021. 2021.
- 8.https://www.ncirs.org.au/sites/default/files/2022-02/COVID-19-history-February%202022_0.pdf.
- 9.Ritchie H., Mathieu E., Rodés-Guirao L., et al. Coronavirus Pandemic (COVID-19) OurWorldInDataorg. 2021;https://ourworldindata.org/covid-cases. [Google Scholar]
- 10.https://view-hub.org/covid-19/effectiveness-studies.
- 11.Hall V., Foulkes S., Insalata F., et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N Engl J Med. 2022;386:1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.https://www.health.nsw.gov.au/Infectious/covid-19/Documents/covid-19-surveillance-report-20211120.pdf.
- 13.https://ourworldindata.org/explorers/coronavirus-data-explorer?facet=none&Interval=7-day+rolling+average&Relative+to+Population=true&Color+by+test+positivity=false&country=∼AUS&Metric=Omicron+variant+%28share%29.
- 14.https://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa.
- 15.https://www.health.nsw.gov.au/Infectious/covid-19/Documents/in-focus/covid-19-vaccination-case-surveillance-051121.pdf.
- 16.Austin P. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. J Clin Epidemiol. 2010;63:46–55. doi: 10.1016/j.jclinepi.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022;386:1712–1720. doi: 10.1056/NEJMoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews N., Stowe J., Kirsebom F., et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchan SA, Chung H, Brown KA, et al. Effectiveness of COVID-19 vaccines against Omicron or Delta infection. medRxiv 2022:2021.12.30.21268565.
- 20.Hansen CH, Schelde AB, Moustsen-Helm IR, et al. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: A Danish cohort study. medRxiv 2021:2021.12.20.21267966.
- 21.Sheikh A., Kerr S., Woolhouse M., et al. Severity of omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): a national cohort study with nested test-negative design. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. medRxiv 2022:2022.03.13.22272308. [DOI] [PMC free article] [PubMed]
- 23.Altarawneh H.N., Chemaitelly H., Hasan M.R., et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N Engl J Med. 2022;386:1288–1290. doi: 10.1056/NEJMc2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suarez Castillo M., Khaoua H., Courtejoie N. Vaccine-induced and naturally-acquired protection against Omicron and Delta symptomatic infection and severe COVID-19 outcomes, France, December 2021 to January 2022. Eurosurveillance. 2022;27:2200250. doi: 10.2807/1560-7917.ES.2022.27.16.2200250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerqueira-Silva T, Shah SA, Robertson C, et al. Waning of mRNA Boosters after Homologous Primary Series with BNT162b2 or ChadOx1 Against Symptomatic Infection and Severe COVID-19 in Brazil and Scotland: A Test-Negative Design Case-Control Study. . http://dxdoiorg/102139/ssrn4082927 2022. [DOI] [PMC free article] [PubMed]
- 26.Chen Y, Tong P, Whiteman N, et al. Immune recall improves antibody durability and breadth to SARS-CoV-2 variants. Science Immunology;0:eabp8328. [DOI] [PMC free article] [PubMed]
- 27.Bates TA, McBride SK, Leier HC, et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Science Immunology 2022;7:eabn8014. [DOI] [PMC free article] [PubMed]
- 28.https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-immunity-and-increasing-population-seroprevalence-rates.
- 29.Feikin D.R., Abu-Raddad L.J., Andrews N., et al. Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the World Health Organization. Vaccine. 2022;40:3516–3527. doi: 10.1016/j.vaccine.2022.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu B., Spokes P., He W., Kaldor J. High risk groups for severe COVID-19 in a whole of population cohort in Australia. BMC Infect Dis. 2021;21:685. doi: 10.1186/s12879-021-06378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.https://www.health.nsw.gov.au/Infectious/covid-19/Documents/covid-19-surveillance-report-20220201.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.