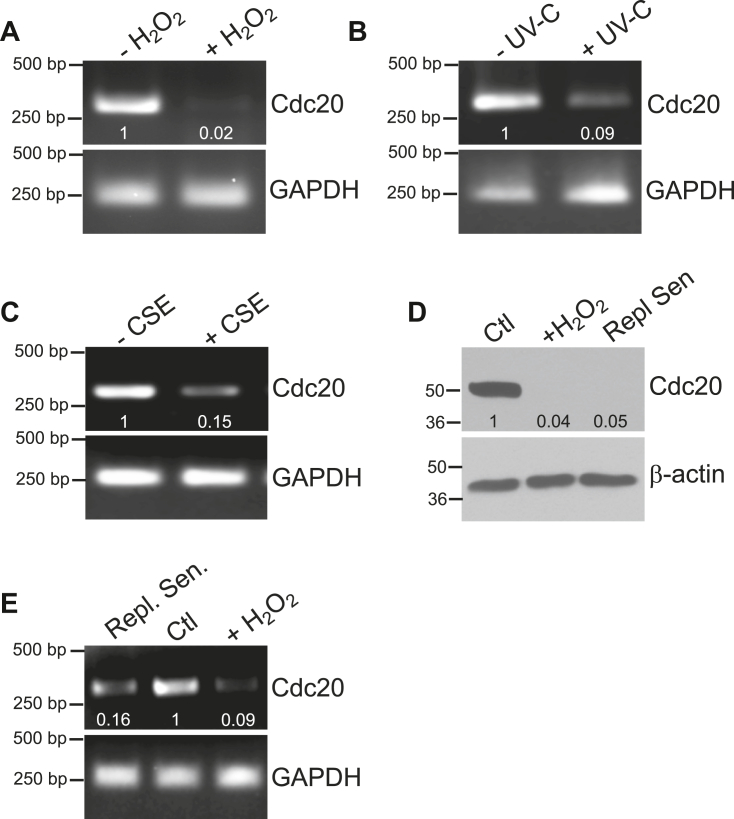
Figure 2.
Cdc20 mRNA is downregulated in stress-induced and replicative senescent WI-38 fibroblasts.A, WI-38 fibroblasts were treated with sublethal hydrogen peroxide (450 μM) for 2 h to induce premature senescence. Cells were washed with PBS and recovered in complete medium for 14 days. Cells were collected and the expression level of Cdc20 mRNA was determined by RT-PCR using primers specific for Cdc20. Untreated cells were used as control. Expression of GAPDH was detected as internal control. B, human WI-38 fibroblasts were treated with sublethal UV-C light (10 J/m2) to induce premature senescence. Cells were then recovered in complete medium for 14 days. Cells were collected and the expression level of Cdc20 mRNA was determined by RT-PCR using primers specific for Cdc20. Untreated cells were used as control. Expression of GAPDH was detected as internal control. C, WI-38 cells were treated with 2% cigarette smoke extracts (CSEs) for 14 days to induce premature senescence. Cells were collected and the expression level of Cdc20 mRNA was determined by RT-PCR using primers specific for Cdc20. Untreated cells were used as control. Expression of GAPDH was detected as internal control. D, lysates from nonsenescent (Ctl) and replicative senescent human WI-38 cells were subjected to immunoblotting analysis using anti-Cdc20 IgGs. Lysate from oxidative stress–induced premature senescent WI-38 cells was used as positive control. Anti-β-actin IgGs were used to show equal total protein loading. Quantification of protein band intensity is shown at the bottom of the blot. E, expression of Cdc20 mRNA in nonsenescent (Ctl) and replicative senescent WI-38 fibroblasts was determined by RT-PCR using primers specific for Cdc20. Cdc20 mRNA expression in oxidative stress–induced senescent WI-38 cells served as positive control. Expression of GAPDH was detected as internal control. Quantification of mRNA band intensity is shown at the bottom of each blot.