Fig. 2.
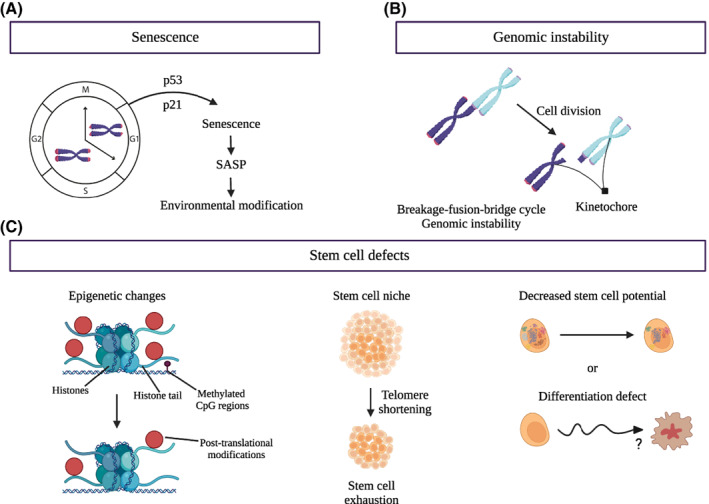
Consequences of telomere shortening. (A) Senescence is referred to as an irreversible exit from the cell cycle. This state can occur via a variety of mechanisms that include eroded telomeres. When telomeres reach a critically short threshold, cells undergo telomere‐induced (or replicative) senescence, which is triggered by p53 and its downstream effector p21, a cyclin‐dependent kinase inhibitor that arrests the cell cycle. Senescent cells can secrete factors that modify neighbouring tissues and cells and enhance aging, via a process termed the senescence‐associated secretory phenotype (SASP). (B) If a cell bypasses senescence and continues to divide, telomere fusions can occur, leading to a cycle of chromosome breakage‐fusion‐bridge formation and genetic instability. (C) Epigenetic modifications are also linked to telomere shortening. Such modifications can influence transcriptional activity and cell fate. In the left image, DNA wrapped around the histones (represented by the green spheres) may contain extensive post‐translational modifications (red sphere) on the histone tail, and the DNA itself can be methylated (small black sphere). Murine embryonic stem cells are particularly sensitive to epigenetic modifications driven by telomere shortening, which can reduce stem cell renewal potential (middle image). Telomere shortening can also affect the ability of stem cells to maintain a functional pluripotent state and/or differentiated state (right image). [Colour figure can be viewed at wileyonlinelibrary.com]
