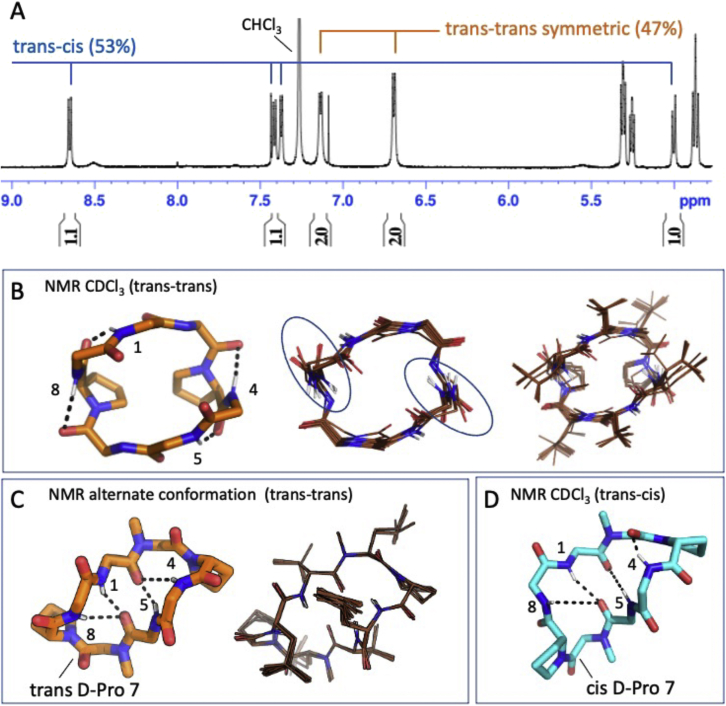
Figure S9.
NMR structures of D8.21 have conformational ambiguity, related to Figure 4
(A) 1D 1H NMR spectrum of D8.21 in CDCl3 collected at 600 MHz and 293 K. Amide 1H peaks for each conformation are indicated.
(B and C) (B) The trans-trans NMR structure (47% population) in CDCl3 that best matches the NMR data. It has an “open” conformation with only surface hydrogen bonds and buried but unsatisfied NHs and is similar to the trans-trans conformation observed in DMSO and DMSO-water. A representative member of this ensemble (left panel) shows four surface hydrogen bonds, whereas the ensemble of 20 structures (backbone—middle panel; backbone plus sidechain—right panel) shows the variability in orientations of the NH donor and carbonyl acceptor conformations along with buried, unsatisfied NHs. (B) An alternate “closed” trans-trans conformation. In order to assess whether the NMR data obtained for D8.21 in CHCl3 could possibly be fit to a more closed conformation with buried hydrogen bonds, we also used Cyana to reassign the ROESY data subject to restraints imposed for the four specific hydrogen bonds observed in the “closed” trans-cis conformation (47% in CHCl3), which are also observed in the designed and predicted low-energy trans-trans state (design model and LE_1 in Figure S8). Although the open trans-trans conformation (B) fits the NMR ROESY and amide temperature coefficient data better than the closed conformation, the alternate closed trans-trans conformation (C) cannot be ruled out. This ambiguity is due primarily to the chemical shift degeneracy that results from the repetitive 4-residue sequence (v∗LpL)2 and the symmetry of this conformation. The assignment of ROEs between degenerate/symmetric chemical shifts has multiple possibilities. For example, the assignment of ROEs to short distances between D-Pro 3 and D-Pro 7 rings in the open conformation is ambiguous because they are indistinguishable from intraresidue D-proline peaks. For the closed conformation, the D-Val 1 and 5 HNs have a characteristic short distance that is not observable in the ROESY data due to their degenerate chemical shifts. The alternative assignments of the ROEs by Cyana are based on preliminary structures and can be influenced by one or two manual restraints guiding it toward one or the other conformation.
(D) The trans-cis conformation determined from NMR data in CHCl3 (53% population).