Figure 1. Patient Disposition in the DERMIS-1 and DERMIS-2 Trials.
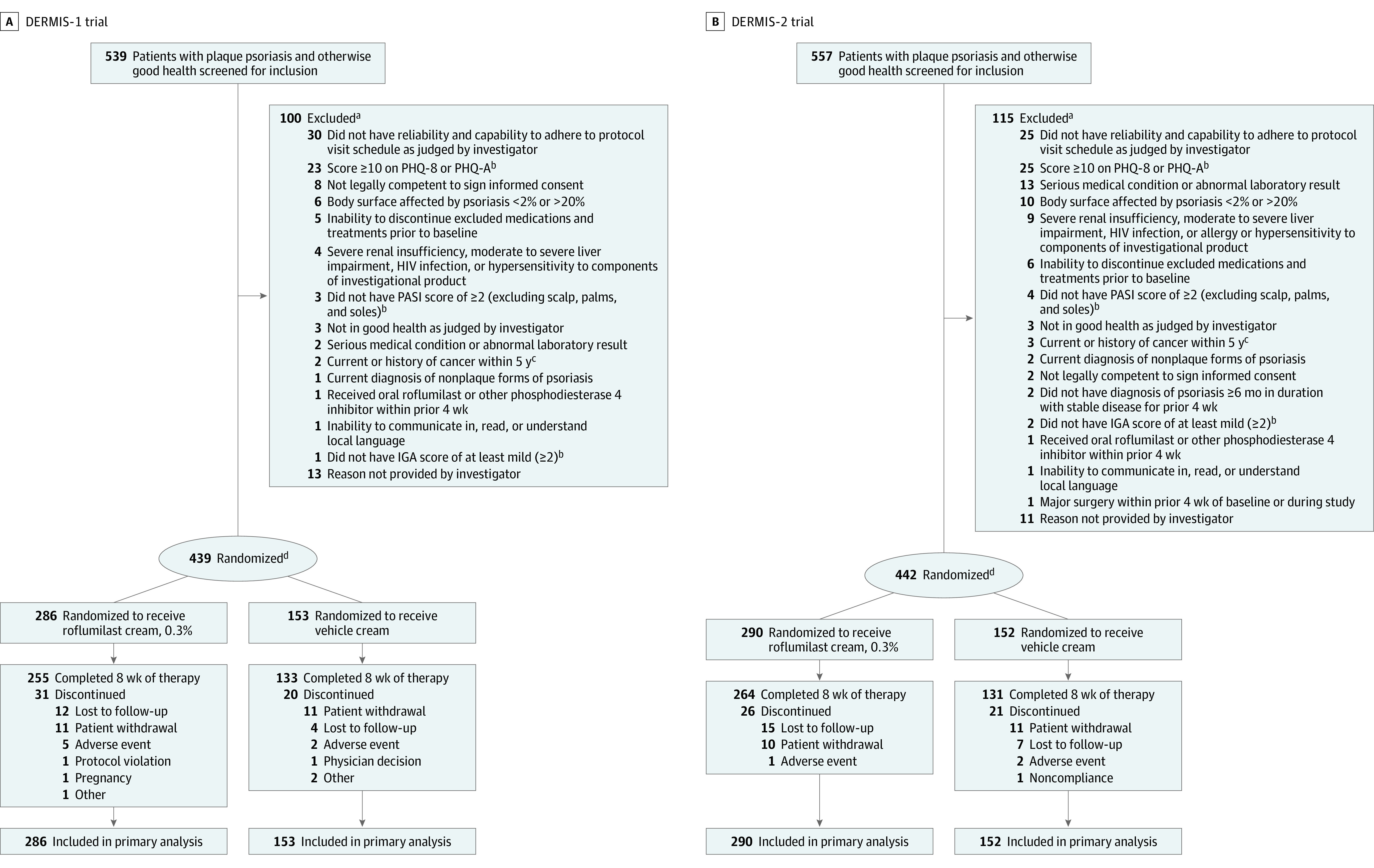
aExcluded participants could have ≥1 reasons for ineligibility/nonenrollment.
bDescriptions of PHQ-8 and modified PHQ-A are in the Methods; descriptions of PASI7 and IGA8 are in Table 1 footnotes.
cExcept fully treated basal cell carcinoma, cutaneous squamous cell carcinoma, or carcinoma in situ of the cervix.
dRandomization was stratified by study site, baseline IGA score (2 vs ≥3), and intertriginous involvement at baseline (I-IGA score >2, yes vs no; I-IGA evaluated on a scale of 0 [clear] to 4 [severe]).
