Key Points
Question
What is the effect of noninvasive ventilation delivered by helmet compared with usual respiratory support (mask noninvasive ventilation, high-flow nasal oxygen, and standard oxygen) on the risk of mortality among adults with acute hypoxemic respiratory failure due to COVID-19?
Findings
In this randomized clinical trial that included 320 adults with acute hypoxemic respiratory failure related to COVID-19, randomization to helmet use compared with usual respiratory support resulted in mortality within 28 days in 27.0% vs 26.1%, respectively. This difference was not statistically significant.
Meaning
Helmet noninvasive ventilation did not significantly reduce 28-day mortality compared with usual respiratory support among patients with acute hypoxemic respiratory failure due to COVID-19 pneumonia; however, interpretation of the findings is limited by imprecision in the effect size estimate.
Abstract
Importance
Helmet noninvasive ventilation has been used in patients with COVID-19 with the premise that helmet interface is more effective than mask interface in delivering prolonged treatments with high positive airway pressure, but data about its effectiveness are limited.
Objective
To evaluate whether helmet noninvasive ventilation compared with usual respiratory support reduces mortality in patients with acute hypoxemic respiratory failure due to COVID-19 pneumonia.
Design, Setting, and Participants
This was a multicenter, pragmatic, randomized clinical trial that was conducted in 8 sites in Saudi Arabia and Kuwait between February 8, 2021, and November 16, 2021. Adult patients with acute hypoxemic respiratory failure (n = 320) due to suspected or confirmed COVID-19 were included. The final follow-up date for the primary outcome was December 14, 2021.
Interventions
Patients were randomized to receive helmet noninvasive ventilation (n = 159) or usual respiratory support (n = 161), which included mask noninvasive ventilation, high-flow nasal oxygen, and standard oxygen.
Main Outcomes and Measures
The primary outcome was 28-day all-cause mortality. There were 12 prespecified secondary outcomes, including endotracheal intubation, barotrauma, skin pressure injury, and serious adverse events.
Results
Among 322 patients who were randomized, 320 were included in the primary analysis, all of whom completed the trial. Median age was 58 years, and 187 were men (58.4%). Within 28 days, 43 of 159 patients (27.0%) died in the helmet noninvasive ventilation group compared with 42 of 161 (26.1%) in the usual respiratory support group (risk difference, 1.0% [95% CI, −8.7% to 10.6%]; relative risk, 1.04 [95% CI, 0.72-1.49]; P = .85). Within 28 days, 75 of 159 patients (47.2%) required endotracheal intubation in the helmet noninvasive ventilation group compared with 81 of 161 (50.3%) in the usual respiratory support group (risk difference, −3.1% [95% CI, −14.1% to 7.8%]; relative risk, 0.94 [95% CI, 0.75-1.17]). There were no significant differences between the 2 groups in any of the prespecified secondary end points. Barotrauma occurred in 30 of 159 patients (18.9%) in the helmet noninvasive ventilation group and 25 of 161 (15.5%) in the usual respiratory support group. Skin pressure injury occurred in 5 of 159 patients (3.1%) in the helmet noninvasive ventilation group and 10 of 161 (6.2%) in the usual respiratory support group. There were 2 serious adverse events in the helmet noninvasive ventilation group and 1 in the usual respiratory support group.
Conclusions and Relevance
Results of this study suggest that helmet noninvasive ventilation did not significantly reduce 28-day mortality compared with usual respiratory support among patients with acute hypoxemic respiratory failure due to COVID-19 pneumonia. However, interpretation of the findings is limited by imprecision in the effect estimate, which does not exclude potentially clinically important benefit or harm.
Trial Registration
ClinicalTrials.gov Identifier: NCT04477668
This randomized clinical trial evaluates whether helmet noninvasive ventilation compared with usual respiratory support reduces mortality in patients with acute hypoxemic respiratory failure due to COVID-19 pneumonia.
Introduction
Noninvasive respiratory support in the form of noninvasive ventilation and high-flow nasal oxygen has been used widely for patients with acute hypoxemic respiratory failure due to COVID-19,1 and data about its effectiveness are emerging.2,3,4 Helmet noninvasive ventilation has been used in patients with COVID-19 with the premise that helmet interface is more effective than mask interface in delivering prolonged treatments with high positive airway pressure because it is associated with fewer air leaks and better fitting for different facial contours.5 Additionally, helmet noninvasive ventilation may be associated with less risk for skin pressure injury, eye irritation, and aerosol generation.6
Helmet ventilation has been in use for 2 decades in certain countries, especially Italy; and its use has increased during the COVID-19 pandemic.7 In March 2020, the US Food and Drug Administration issued Emergency Use Authorizations to several manufacturers for helmet use in acute hypoxemic respiratory failure from COVID-19.8 Supporting data for helmet noninvasive ventilation are mainly based on non–COVID-19 populations. A systematic review that included observational studies and randomized clinical trials showed that helmet compared with mask noninvasive ventilation might be associated with statistically significant lower mortality and endotracheal intubation; however, the effect of helmet noninvasive ventilation compared with high-flow nasal oxygen was uncertain.9 A network meta-analysis of randomized clinical trials published before May 2020 found that helmet noninvasive ventilation might significantly reduce mortality and endotracheal intubation in non–COVID-19 populations compared with mask noninvasive ventilation, high-flow nasal oxygen, and standard oxygen, although the evidence was graded as low quality.10 Therefore, the effect of helmet noninvasive ventilation on mortality in COVID-19 populations remains unclear.
The objective of this study was to evaluate whether helmet noninvasive ventilation compared with usual respiratory support would reduce 28-day all-cause mortality in patients with acute hypoxemic respiratory failure due to COVID-19.
Methods
Trial Design and Oversight
The Helmet-COVID trial was an investigator-initiated, pragmatic, multicenter randomized clinical trial that was conducted in 7 sites in Saudi Arabia and 1 in Kuwait (eTable 1 in Supplement 2).11,12 Details of the trial design have been reported previously11,12 and are available in the trial protocol and statistical analysis plan in Supplement 1. Helmet noninvasive ventilation was introduced in June 2020 in Saudi Arabia as part of the COVID-19 pandemic response to augment the capacity for respiratory support. A national treatment protocol and training program were developed. For each new site joining the study, a training session was provided along with a training video, posters, and written protocol (eTable 2 and eFigure 1 in Supplement 2). The trial protocol was designed by the management committee and approved by the institutional review boards at all participating sites. A priori or deferred informed written or witnessed oral consent was obtained from all patients or surrogates in accordance with local approvals. The study followed the CONSORT reporting guidelines.
Patients
We enrolled adult patients admitted to the intensive care unit with acute hypoxemic respiratory failure (the ratio of Pao2 to fraction of inspired oxygen [Fio2] <200 despite supplemental oxygen with a flow rate ≥10 L/min) and suspected or confirmed COVID-19 pneumonia by reverse transcriptase–polymerase chain reaction. A full list of eligibility criteria is provided in eTable 3 in Supplement 2.
Randomization
Eligible patients were randomly assigned in a 1:1 ratio to either the helmet noninvasive ventilation group or the usual respiratory support group. A centralized computer-generated randomization system with undisclosed variable block sizes of 4 and 6 was used. Randomization was stratified by center.
Intervention
In the helmet noninvasive ventilation group, a helmet (Subsalve) was applied according to a written protocol (eTable 2 in Supplement 2). Helmet noninvasive ventilation was delivered in pressure support mode through an intensive care ventilator, with initial settings of pressure support of 8 to 10 cm H2O and positive end-expiratory pressure (PEEP) of 10 cm H2O with Fio2 of 1.0, targeting a flow rate of at least 50 L/min with an inspiratory rise time of 50 ms and end flow/cycling off of 50% of maximal inspiratory flow.13 If needed, PEEP was increased by 2 cm H2O every 3 minutes to achieve oxygen saturation by pulse oximetry greater than or equal to 90% on Fio2 less than or equal to 0.6, and pressure support was increased by 2 cm H2O every 3 minutes to achieve respiratory rate less than or equal to 25/min and disappearance of accessory muscle activity. The maximal allowed airway pressure (pressure support plus PEEP) was 30 cm H2O.13 Interruptions of the helmet were avoided or kept at a minimum at least in the first 48 hours.14 Dexmedetomidine infusion was allowed to improve comfort with helmet noninvasive ventilation. Other intravenous sedatives such as benzodiazepines or intravenous narcotics were not permitted. If a patient continued to be intolerant to the helmet, he or she was treated according to the usual respiratory support.
In the usual respiratory support group, patients were treated according to the clinical practices of each site, which included mask noninvasive ventilation, high-flow nasal oxygen, and standard oxygen.
Cointerventions
The decision to use endotracheal intubation in both groups was at the discretion of the treating team, although criteria for endotracheal intubation were provided as a guide (eFigure 1 and eTable 2 in Supplement 2). Other aspects of critical care management, including therapeutics for COVID-19, were provided in accordance with local protocols.
Data Collection
Patients’ demographic characteristics, severity of illness, arterial blood gas measurements, and respiratory support were documented at enrollment. Data were recorded on the intervention and cointerventions in the intensive care unit up to 28 days after randomization, including the respiratory support modalities, vasopressor therapy, kidney replacement therapy, corticosteroid therapy, use of immune modulators, COVID-19 antiviral therapy, and fluid balance. The number of hours of helmet noninvasive ventilation and other respiratory support modalities was calculated and the settings of the helmet and mask noninvasive ventilation (pressure support level and PEEP) were recorded. The number of patients for whom helmet noninvasive ventilation was removed because of intolerance was documented. Respiratory rate, the ratio of oxygen saturation by pulse oximetry to Fio2, dyspnea and device-related discomfort (measured through 0-10 visual analog scale scores) (eFigure 2 in Supplement 2), and Paco2 (among patients who had serial arterial blood gas measurements) were documented during the first 4 days.15
Outcomes
The primary outcome was 28-day all-cause mortality. There were 12 prespecified secondary outcomes (10 reported here), including endotracheal intubation within 28 days, intensive care unit mortality, hospital mortality, intensive care unit–free days, invasive ventilation–free days, kidney replacement therapy–free days, and vasopressor-free days at day 28 (eTable 4 in Supplement 2). Adverse events included skin pressure injuries, barotrauma, and serious adverse events (cardiovascular events and device malfunction). In addition, mortality and quality of life measured with EuroQoL 5D-5L at day 180 were planned to be reported separately. For patients who received invasive ventilation, we recorded time to endotracheal intubation, invasive mechanical ventilation settings, and the use of oxygen rescue therapies. Hospital length of stay was evaluated as a post hoc secondary outcome.
Sample Size Calculation
The baseline 28-day mortality was estimated to be 40% according to early reports that were available at study planning.16,17 The treatment effect of risk difference was estimated to be –15% according to available data from a network meta-analysis in non–COVID-19 populations; helmet noninvasive ventilation significantly reduced mortality compared with standard oxygen (risk difference, –19%; 95% CI, –37% to –9%), high-flow nasal oxygen (risk difference, –15%; 95% CI, –34% to –5%), and mask invasive ventilation (risk difference, –13%; 95% CI, –27% to –5%).10 The planned sample size of 320 patients was estimated to provide 80% power to detect a reduction in the primary outcome from 40% to 25%, accounting for a 5% loss to follow-up.16,18 The data and safety monitoring board reviewed data after 110 and 220 patients had completed follow-up and recommended continuing with enrollment. The O’Brien-Fleming method was used to account for α spending and considered P < .048 for the final analysis to be significant.
Statistical Analysis
The primary analyses were conducted with patients analyzed according to their randomization group; the primary analysis population included all randomized patients except those identified as ineligible after randomization. The per-protocol population consisted of all randomized patients who received the allocated intervention (helmet noninvasive ventilation for ≥1 hour in the helmet noninvasive ventilation group and no helmet noninvasive ventilation in the usual respiratory support group). There were no missing outcomes data.
The primary outcome was compared between groups in the primary analysis population with a χ2 test and the result was reported as risk difference and relative risk with 95% CIs. As a secondary analysis, the effect of the intervention on the primary outcome was evaluated in an unadjusted Cox proportional hazards model. The proportional hazards assumption was evaluated with the supremum test, which indicated that the proportionality assumption was met (P = .99). Additionally, the effect of the intervention on the primary outcome was evaluated in a generalized linear mixed model adjusting for the following prespecified covariates: respiratory support at baseline (mask noninvasive ventilation vs others), baseline Pao2:Fio2 ratio, body mass index (>30 vs ≤30, calculated as weight in kilograms divided by height in meters squared), age, APACHE II score, and time (being enrolled in the first or second half of the study to account for changes in outcomes during the COVID-19 pandemic) and enrollment center as random effect. The time-to-event distributions were compared between the helmet noninvasive ventilation and usual respiratory support groups, using Kaplan-Meier curves and log-rank tests. Similar analyses were conducted in the per-protocol population.
We conducted analyses of secondary outcomes and subgroup analyses in the primary analysis population only. We compared the primary outcome in the following prespecified subgroups: Pao2:Fio2 ratio of 101 to 200 and Pao2:Fio2 ratio less than or equal to 100, body mass index of greater than 30 and less than or equal to 30, older than 65 years and aged 65 years or younger, APACHE II score higher and lower than the median value, and respiratory support at enrollment with mask noninvasive ventilation and other types (eTable 5 in Supplement 2). Additionally, we conducted post hoc subgroup analyses according to the center experience with helmet noninvasive ventilation before the trial (centers with >50 vs ≤50 patients treated before the trial), the center experience with mask noninvasive ventilation before the trial (≥20 years vs 10-19 years), and baseline Paco2 (>35 mm Hg vs ≤35 mm Hg) (eTable 5 in Supplement 2). Heterogeneity of the intervention effects on the primary outcome among subgroups was evaluated with test of interaction using log binomial regression. Analyses of secondary outcomes, prespecified subgroups, and post hoc subgroups were adjusted for multiple testing with the false discovery rate.19 There was no imputation for missing values. Tests were 2-sided and at the 5% significance level and were conducted with SAS version 9.4 (SAS Institute).
Results
Patients
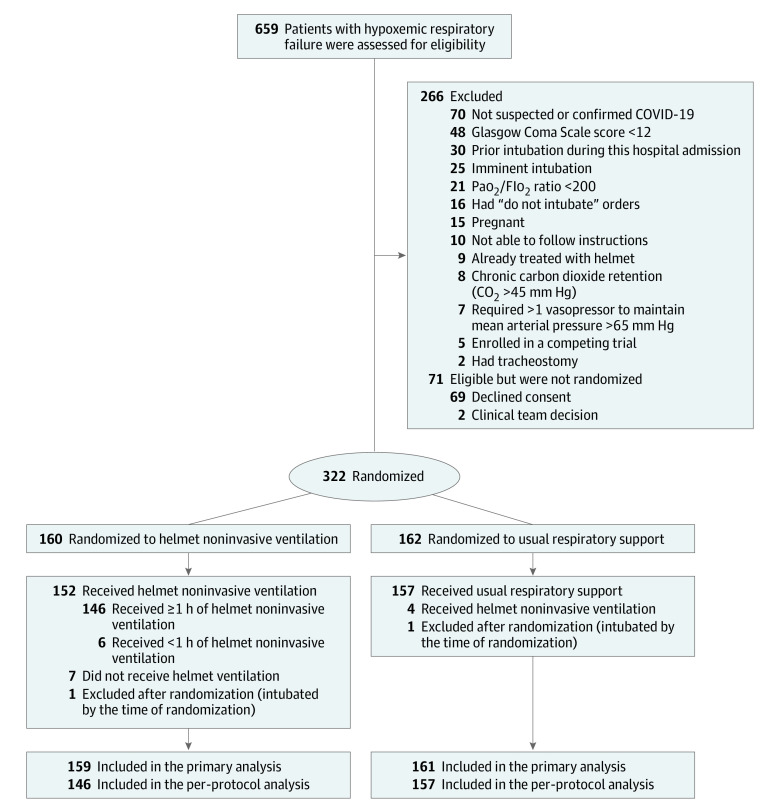
Characteristics of the participating sites including the experience with helmet noninvasive ventilation are summarized in eTable 1 in Supplement 2. From February 8, 2021, to November 16, 2021, we assessed 659 patients for eligibility. Among 322 patients who were randomized, 320 were included in the primary analysis; 159 were assigned to the helmet noninvasive ventilation group and 161 to the usual respiratory support group (Figure 1; eTables 1 and 6 in Supplement 2). Median age was 58 years, 133 patients were women (41.6%), and 187 were men (58.4%). All 320 patients completed the study; the final follow-up date for the primary outcome was December 14, 2021. Most randomized patients had confirmed COVID-19 by polymerase chain reaction test for SARS-CoV-2 from respiratory specimens; the results of positive tests were known either at randomization or soon after, totaling 157 of 159 patients (98.7%) in the helmet noninvasive ventilation group and 160 of 161 patients (99.4%) in the usual respiratory support group (Table 1).
Figure 1. Screening, Randomization, and Participant Flow in the Helmet-COVID Randomized Clinical Trial.
Fio2 indicates fraction of inspired oxygen. Randomization was stratified according to site.
Table 1. Baseline Characteristics in the Primary Analysis Population in the Helmet Noninvasive Ventilation and Usual Respiratory Support Groups.
| Characteristica | No. (%) | |
|---|---|---|
| Helmet noninvasive ventilation (n = 159) | Usual respiratory support (n = 161) | |
| Age, median (IQR), y | 57 (47-66) | 59 (50-67) |
| Sex | ||
| Women | 57 (35.8) | 76 (47.2) |
| Men | 102 (64.2) | 85 (52.8) |
| BMI, median (IQR) | 30.1 (26.7-35.2) | 29.9 (26.7-34.7) |
| Location before ICU admission | ||
| Emergency department | 86 (54.1) | 86 (53.4) |
| Hospital ward | 46 (28.9) | 54 (33.5) |
| Transfer from outside hospital ICU or ward | 27 (17.0) | 21 (13.0) |
| APACHE II score, median (IQR)b | 13 (10-16) | 14 (10-17) |
| SOFA score, median (IQR)b | 2 (2-4) | 3 (2-4) |
| Comorbiditiesc | ||
| Any chronic comorbidityc | 108 (67.9) | 117 (72.7) |
| Diabetes | 86 (54.1) | 95 (59.0) |
| Chronic cardiac disease | 57 (35.8) | 52 (32.3) |
| Chronic pulmonary disease | 24 (15.1) | 19 (11.8) |
| Chronic kidney disease with dialysis | 8 (5.0) | 8 (5.0) |
| Malignancy | 8 (5.0) | 10 (6.2) |
| Confirmed COVID-19 at enrollmentd | 156 (98.1) | 157 (97.5) |
| Physiologic parameters before randomization, median (IQR) | ||
| Pao2, mm Hg | 60 (52-70) | 60 (54-70) |
| Fio2 | 80 (70-100) | 80 (60-100) |
| Pao2:Fio2 ratio | 73 (60-93) | 76 (61-111) |
| Pco2, mm Hg | 36 (32-39) | 35 (32-39) |
| Hco3, mEq/L | 24 (22-26) | 24 (22-26) |
| pH | 7.43 (7.40-7.46) | 7.43 (7.40-7.46) |
| Quadrants with infiltrates on chest radiograph, No. (IQR)e | 4 (3-4) | 4 (3-4) |
| Respiratory support at baseline | ||
| High-flow nasal oxygen | 93 (58.5) | 78 (48.4) |
| Mask noninvasive ventilation | 45 (28.3) | 64 (39.8) |
| Standard oxygenf | 21 (13.2) | 19 (11.8) |
| Respiratory rate, median (IQR), breaths/min | 31 (27-35) | 30 (26-33) |
| Awake prone positioning | 30 (18.9) | 26 (16.1) |
| Days from onset of symptoms to emergency department visit, median (IQR) | 6 (3-8) | 5 (3-8) |
| Days from onset of symptoms to ICU admission, median (IQR) | 8 (5-10) | 7 (5-11) |
| Days from ICU admission to randomization, median (IQR) | 2 (1-2) | 2 (1-2) |
| Organ support | ||
| Vasopressors | 13 (8.2) | 12 (7.5) |
| Kidney replacement therapy for acute kidney injury | 0 | 3 (1.9) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); Fio2, fraction of inspired oxygen; ICU, intensive care unit; SOFA, Sequential [Sepsis-related] Organ Failure Assessment.
The number of patients for each variable is the total number of patients in the respective group. There were no missing values. Percentages may not total to 100 because of rounding. Additional details on baseline characteristics are provided in eTable 8 in Supplement 2.
The APACHE II score measures severity of illness and is based on age, medical history, and physiologic parameters. The score ranges from 0 to 71, with higher scores indicating more severe disease and higher risk of death. The SOFA score ranges from 0 to 24, with higher scores indicating a greater degree of organ dysfunction.
Data on comorbidities were obtained from the medical record. Other comorbidities included mild, moderate, or severe liver disease; chronic neurologic disease, hemiplegia or paraplegia, or dementia; and AIDS/HIV and rheumatologic diseases (eTable 8 in Supplement 2).
COVID-19 infection was confirmed by polymerase chain reaction test for SARS-CoV-2 from respiratory specimens. After the results of testing were obtained at enrollment, additional patients were confirmed to have COVID-19, totaling 157 of 159 (98.7%) in the helmet noninvasive ventilation group and 160 of 161 (99.4%) in the usual respiratory support group. No patients received a diagnosis by rapid antigen test or solely by clinical criteria.
The number of quadrants with infiltrates on chest radiograph was reported as determined by research coordinator review of images and confirmed subsequently with radiology reporting.
Standard oxygen included oxygen delivery via any device other than high-flow nasal cannula or noninvasive ventilation regardless of the Fio2 delivered to the patient.
The characteristics of the patients at baseline did not differ significantly between the 2 trial groups (Table 1; eTable 7 in Supplement 2). Median Pao2:Fio2 ratio on enrollment was 73 (IQR, 60-93) in the helmet noninvasive ventilation group and 76 (IQR, 61-111) in the usual respiratory support group.
Intervention
In the primary analysis population, helmet noninvasive ventilation was used for 152 of the 159 patients (95.6%) in the helmet noninvasive ventilation group and 4 of the 161 patients (2.5%) in the control group (Table 2). In the first 48 hours, patients in the helmet noninvasive ventilation group were treated with helmet noninvasive ventilation for a median of 34 hours (IQR, 15-46 hours) and with mask noninvasive ventilation for 0 hours (IQR, 0-5 hours) compared with patients in usual respiratory support, who received helmet treatment for 0 hours (IQR, 0-0 hours) and mask noninvasive ventilation for 14 hours (IQR, 0-26.5 hours). A total of 58 of 159 patients (36.5%) in the helmet noninvasive ventilation group discontinued the helmet because of intolerance after 20.5 hours of use (IQR, 3-48 hours). Further details regarding respiratory support are presented in eTable 8 in Supplement 2. Cointerventions including vasopressors, kidney replacement therapy, corticosteroids, and tocilizumab were not different between the 2 groups (Table 1; eTable 8 in Supplement 2).
Table 2. Summary of Interventions and Cointerventions in the Primary Analysis Population.
| Variablea | No. (%) | |
|---|---|---|
| Helmet noninvasive ventilation (n = 159) | Usual respiratory support (n = 161) | |
| Helmet NIV use during the 28-d study period | ||
| No. of patients | 152 (95.6) | 4 (2.5) |
| Total duration of helmet use, median (IQR), h | 43 (19.5-70.5) | 0 (0-0) |
| Noninvasive respiratory support in the first 48 h | ||
| Helmet NIV | ||
| No. of patients | 151 (95.0) | 3 (1.9) |
| Duration of use, median (IQR), h | 34 (15-46) | 0 (0-0) |
| Mask NIV | ||
| No. of patients | 43 (27.0) | 111 (68.9) |
| Duration of use, median (IQR), h | 0 (0-5) | 14 (0-26.5) |
| Helmet or mask NIV | ||
| No. of patients | 154 (96.9) | 111 (68.9) |
| Duration of use, median (IQR), h | 40 (24-48) | 14.0 (0-27) |
| High-flow nasal oxygen | ||
| No. of patients | 91 (57.2) | 122 (75.8) |
| Duration of use, median (IQR), h | 3 (0-15) | 23 (4-39) |
| Standard oxygen | ||
| No. of patients | 25 (15.7) | 33 (20.5) |
| Duration of use, median (IQR), h | 0 (0-0) | 0 (0-0) |
| Noninvasive ventilation settings (via helmet or mask), day 1 | ||
| Highest pressure support level, median (IQR) [No.], cm H2O | 8 (8-10) [152] | 8 (0-10) [102] |
| Highest PEEP, median (IQR) [No.], cm H2O | 10 (10-10) [152] | 10 (8-10) [102] |
| Cointerventions during the study period | ||
| Vasopressors/inotropes | 74 (46.5) | 79 (49.1) |
| Dexmedetomidine use during noninvasive respiratory supportb | 69 (43.4) | 41 (25.5) |
| Awake prone positioning | 42 (26.4) | 49 (30.4) |
| Kidney replacement therapy | 21 (13.2) | 20 (12.4) |
| COVID-19 therapeutics | ||
| Corticosteroids | 159 (100.0) | 161 (100.0) |
| Tocilizumab | 104 (65.4) | 80 (49.7) |
Abbreviations: NIV, noninvasive ventilation; PEEP, positive end-expiratory pressure.
All calculations are provided for all patients in each group, with the exception of noninvasive ventilation (helmet or mask noninvasive ventilation) settings, which were provided for patients receiving noninvasive ventilation (helmet or mask noninvasive ventilation). Additional details on the interventions and cointerventions are provided in eTable 8 in Supplement 2.
Dexmedetomidine was used for comfort and to improve compliance; benzodiazepines and other intravenous sedatives were not used.
Primary Outcome
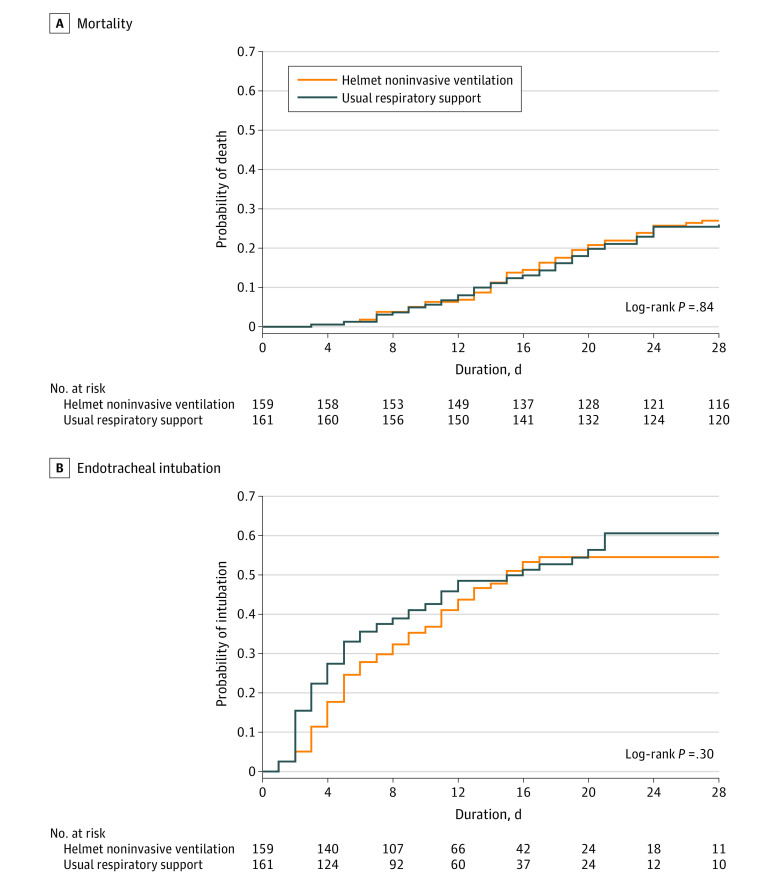
Death from any cause within 28 days occurred in 43 of 159 patients (27.0%) and 42 of 161 patients (26.1%) in the helmet noninvasive ventilation and usual respiratory support groups, respectively (risk difference, 1.0% [95% CI, −8.7% to 10.6%]; relative risk, 1.04 [95% CI, 0.72-1.49]; P = .85). Secondary analyses of the primary outcome in the primary analysis population and the per-protocol population were consistent with the main analysis (Table 3; Figure 2; eTable 9 and eFigure 3 in Supplement 2).
Table 3. Primary and Secondary Outcomes.
| Variablea | No. (%) | Risk difference (95% CI)b | Effect, β estimate (95% CI) | P value | FDR | |
|---|---|---|---|---|---|---|
| Helmet noninvasive ventilation (n = 159) | Usual respiratory support (n = 161) | |||||
| Primary outcome (28-d mortality)c | ||||||
| Primary analysis | 43 (27.0) | 42 (26.1) | 1.0 (−8.7 to 10.6) | Relative risk, 1.04 (0.72 to 1.49) | .85 | |
| Per-protocol analysis | 41/146 (28.1) | 41/157 (26.1) | 2.0 (−8.1 to 12.0) | Relative risk, 1.08 (0.74 to 1.56) | .70 | |
| Secondary outcomes | ||||||
| ICU mortalityd | 56 (35.2) | 60 (37.3) | −2.0 (−12.6 to 8.5) | Relative risk, 0.95 (0.71 to 1.26) | .70 | 0.91 |
| Hospital mortalityd | 61 (38.4) | 64 (39.8) | −1.4 (−12.1 to 9.3) | Relative risk, 0.97 (0.73 to 1.27) | .80 | 0.91 |
| ICU-free days at day 28, median (IQR)e | 12 (0 to 20) | 8 (0 to 19) | Median difference, 4.0 (−2.8 to 10.8) | 0.08 (−0.32 to 0.48) | .70 | 0.91 |
| Mechanical ventilation–free days at day 28, median (IQR)e | 28 (0 to 28) | 23 (0 to 28) | Median difference, 5.0 (−2.6 to 12.6) | 0.05 (−0.28 to 0.38) | .76 | 0.91 |
| Kidney replacement–free days at day 28, median (IQR)e | 28 (0 to 28) | 28 (0 to 28) | −0.02 (−0.33 to 0.30) | .92 | 0.92 | |
| Vasopressor-free days at day 28, median (IQR)e | 28 (0 to 28) | 25 (0 to 28) | Median difference, 3.0 (−2.8 to 8.8) | 0.05 (−0.27 to 0.37) | .76 | 0.91 |
| Hospital LOS, median (IQR), df | 18 (11 to 26) | 17 (11 to 30) | Median difference, 1.0 (−2.1 to 4.1) | −0.16 (−0.33 to 0.00) | .05 | 0.42 |
| Endotracheal intubation | 75 (47.2) | 81 (50.3) | −3.1 (−14.1 to 7.8) | Relative risk, 0.94 (0.75 to 1.17) | .57 | 0.91 |
| Time to intubation, median (IQR) [No.], d | 5 (4 to 10) [75] | 4 (2 to 8) [81] | ||||
Abbreviations: FDR, false discovery rate; Fio2, fraction of inspired oxygen; ICU, intensive care unit; LOS, length of stay; PEEP, positive end-expiratory pressure.
The number of patients for each variable is the total number of patients in the respective group unless otherwise specified. Categorical outcomes were compared with a χ2 test, and the results were reported as risk difference and relative risk with 95% CIs. Continuous outcomes were compared with generalized linear mixed models and the results were reported as β estimates with 95% CI. Denominator of the percentage is the total number of subjects in each group. False discovery rate was used to adjust for multiple testing for the analyses of the following secondary outcomes: ICU mortality, hospital mortality, ICU-free days, mechanical ventilation–free days, kidney replacement–free days, vasopressor-free days, endotracheal intubation, and hospital LOS (hospital LOS was a post hoc secondary outcome).
Risk differences were expressed as percentages.
Secondary analyses of the primary outcome in the primary analysis population and per-protocol population are reported in eTable 9 in Supplement 2.
ICU and hospital mortality are defined as death in the index ICU admission or hospital admission, censored by day 180.
ICU-free days, mechanical ventilation–free days, kidney replacement–free days, and vasopressor-free days are calculated according to 28-day observation.
Hospital LOS was a post hoc secondary outcome.
Figure 2. Kaplan-Meier Time-to-Event Curves for Mortality and Endotracheal Intubation in the Helmet Noninvasive Ventilation and Usual Respiratory Support Groups.
All patients were observed to event or 28 days.
Secondary Outcomes and Adverse Events
There were no significant differences between the 2 groups in any of the prespecified secondary end points (Table 3; eTable 10 in Supplement 2). Within 28 days, 75 of 159 patients (47.2%) required endotracheal intubation in the helmet noninvasive ventilation group compared with 81 of 161 patients (50.3%) in the usual respiratory support group (risk difference, −3.1% [95% CI, −14.1% to 7.8%]; relative risk, 0.94 [95% CI, 0.75-1.17]). For patients who received invasive ventilation, the time to endotracheal intubation, invasive mechanical ventilation settings, and the use of oxygen rescue therapies were not significantly different between the 2 groups. There were no significant differences in serial respiratory rate, ratio of oxygen saturation by pulse oximetry to Fio2, dyspnea or device discomfort visual analog scale scores, Sequential [Sepsis-related] Organ Failure Assessment (SOFA) scores, and Paco2 level between the 2 groups (eFigure 4 in Supplement 2). Barotrauma occurred in 30 of 159 patients (18.9%) in the helmet noninvasive ventilation group and 25 of 161 (15.5%) in the usual respiratory support group within the 28 days of enrollment. Skin pressure injury occurred in 5 of 159 patients (3.1%) in the helmet noninvasive ventilation group and 10 of 161 (6.2%) in the usual respiratory support group (Table 4; eTable 10 in Supplement 2). There were 2 serious adverse events in the helmet noninvasive ventilation group and 1 in the usual respiratory support group (eTable 11 in Supplement 2).
Table 4. Mechanical Ventilation Parameters, Therapies, and Adverse Events.
| Helmet noninvasive ventilation (n = 159) | Usual respiratory support (n = 161) | |
|---|---|---|
| Mechanical ventilation parameters in the first 24 h of intubation, median (IQR) [No.] | ||
| Peak pressure, cm H2O | 32 (30 to 35) [70] | 32 (29 to 34) [76] |
| Plateau pressure, cm H2O | 30 (27 to 31) [53] | 29 (26 to 30) [57] |
| PEEP, cm H2O | 12 (10 to 14) [74] | 12 (10 to 14) [78] |
| Fio2, % | 100 (65 to 100) [74] | 100 (80 to 100) [77] |
| Tidal volume, mL | 400 (350 to 436) [71] | 390 (350 to 400) [74] |
| Respiratory rate, breaths/min | 30 (26 to 34) [74] | 30 (25 to 32) [77] |
| Therapies received during invasive mechanical ventilation | ||
| Neuromuscular blocker infusion | 52 (32.7) | 53 (32.9) |
| Prone positioning | 41 (25.8) | 53 (32.9) |
| Recruitment maneuvers | 17 (10.7) | 14 (8.7) |
| Inhaled nitric oxide | 15 (9.4) | 12 (7.5) |
| Tracheostomy | 11 (6.9) | 17 (10.6) |
| Extracorporeal membrane oxygenation | 4 (2.5) | 2 (1.2) |
| Adverse events | ||
| Barotraumaa | 30 (18.9) | 25 (15.5) |
| Skin pressure injury at nose, face, neck, and axillae (highest stage during intervention period) | 5 (3.1) | 10 (6.2) |
| Serious adverse eventsb | ||
| Cardiovascular events | 2 (1.3) | 1 (0.6) |
| Device complication (helmet deflation) | 0 | 0 |
Abbreviations: Fio2, fraction of inspired oxygen; PEEP, positive end-expiratory pressure.
Barotrauma includes pneumothorax, mediastinal air, and subcutaneous emphysema. Barotrauma was documented for the 28 days of enrollment, including the time of invasive mechanical ventilation.
Serious adverse events included adverse events that were considered to be related to the study interventions but were not captured as one of the other study outcomes. These serious adverse events included 2 patients who experienced cardiac arrest in the helmet noninvasive ventilation group and 1 who experienced ST-segment elevation myocardial infarction in the usual respiratory support group.
Subgroup Analysis and Post Hoc Analyses
There was no statistically significant heterogeneity of treatment effect between the 2 study groups across any of the prespecified subgroups by Pao2:Fio2 ratio, body mass index, age, APACHE II score, and respiratory support at enrollment (eFigure 5 in Supplement 2). There was no statistically significant difference in the hospital length of stay between the 2 study groups. There was no statistically significant heterogeneity of treatment effect between the 2 study groups across any of the post hoc subgroups by center experience with helmet noninvasive ventilation before the trial, center experience with mask noninvasive ventilation before the trial, and baseline Paco2 level (eFigure 6 in Supplement 2).
Discussion
Helmet noninvasive ventilation did not significantly reduce 28-day mortality compared with usual respiratory support among patients with acute hypoxemic respiratory failure due to COVID-19 pneumonia. However, interpretation of the findings is limited by imprecision in the effect estimate, which does not exclude potentially clinically important benefit or harm.
In this pragmatic trial, noninvasive respiratory support in the usual respiratory support group was not restricted to a single modality as used in other trials. Patients in the usual respiratory support group received noninvasive respiratory support at the discretion of the treating teams, allowing the alternate use of mask noninvasive ventilation, high-flow nasal oxygen, or standard oxygen according to clinical response. This approach permitted flexibility in managing such a heterogeneous condition that might evolve during the course of days and was more reflective of usual clinical practice.20,21
In this trial, high intervention fidelity was achieved, with 152 of the 159 patients (95.6%) in the helmet noninvasive ventilation group receiving the allocated intervention; PEEP levels and duration of use were comparable to or exceeded that of other reports.13,14 The use of helmet noninvasive ventilation in the usual respiratory support group was infrequent, occurring for only 4 of the 161 patients (2.5%). Nevertheless, the study highlights the intolerance of some patients for helmet noninvasive ventilation. Of patients who initially agreed to helmet noninvasive ventilation, 4.6% (7/159) declined it. An additional 36.5% of patients (58/159) discontinued helmet noninvasive ventilation because of reported intolerance, although this occurred after 20.5 hours of use (IQR, 3-48 hours).
The lack of a statistically significant benefit of helmet noninvasive ventilation compared with usual respiratory support in the study could be related to several factors. First, the observed 28-day mortality in the study population was lower than the estimate used for sample size calculation, reflecting the declining mortality due to COVID-19 during the pandemic and resulting in reduced study power. The sample size calculation assumed a risk difference of −15%; therefore, the trial results do not exclude the possibility of a lesser but clinically important treatment effect (a mortality reduction of as much as 8.7% or increase of as much as 10.6%). Second, pressure support and PEEP levels in the usual respiratory support were similar to those in the helmet noninvasive ventilation group. In one trial (n = 83), helmet noninvasive ventilation resulted in fewer endotracheal intubations and lower mortality compared with mask noninvasive ventilation provided with lower PEEP.13 One study found that helmet used with the same pressure support, PEEP, and pressurization time (0.2 seconds) as with the mask settings resulted in a greater inspiratory muscle effort and worsened patient-ventilator synchrony, but this difference was abolished by 50% increases in both pressure support and PEEP compared with the mask settings, together with the shortest possible pressurization time (ie, 0.05 seconds).22,23 In the current study, the shortest possible pressurizing time was used with helmet noninvasive ventilation, which would likely explain the similar respiratory rates and dyspnea and device discomfort in the 2 groups. Whether higher positive pressure with helmet noninvasive ventilation would have resulted in improved outcomes remains to be determined. Third, the ability of helmet noninvasive ventilation in reducing self-inflicted lung injury might have been negated, at least in some patients, by inducing large tidal volumes, which is inherently challenging to monitor with helmet treatment. We used levels of pressure support that were comparable to what had been used in other trials, and the Paco2 levels were similar in the 2 groups among patients who had serial arterial blood gas measurements. Fourth, the apparent discordance between the results of the current study and 2 other trials may be related to the control group selection.13,14 One trial compared helmet noninvasive ventilation with high-flow nasal oxygen alone; the other, with mask noninvasive ventilation with lower PEEP than what was used in the control group in the current study.13,14 Fifth, the lack of difference in the current study may be related to the study population with COVID-19. Further work is needed to evaluate whether helmet noninvasive ventilation effects vary across patient populations. Sixth, the effect of helmet noninvasive ventilation may depend on participating sites’ experience with noninvasive ventilation in general and in helmet noninvasive ventilation in particular. However, we did not observe a statistically significant subgroup effect according to site experience.
The study included patients with severe acute hypoxemic respiratory failure due to COVID-19, with low Pao2:Fio2 ratio on a median Fio2 of 0.8 at randomization. As is typical of COVID-19 patients, most of them initially had single-organ failure, as reflected by the rare need for nonrespiratory organ support at randomization and low APACHE II and SOFA scores. This characteristic represents an optimal window for the use of noninvasive respiratory support. Subsequently, nonrespiratory organ involvement developed in many patients, as reflected by the number of patients who required vasopressors and kidney replacement therapy and by the increasing SOFA scores during the study period.
Limitations
This study has several limitations. First, the sample size may have been inadequate to detect a clinically important treatment effect, as highlighted earlier. Second, the treatment allocation could not be blinded to patients or caregivers because of the nature of the intervention. Third, the study design did not allow direct comparison between helmet noninvasive ventilation and each modality in usual respiratory support. Fourth, given the pandemic situation, there was a short time to train centers on helmet use, a therapy that has a learning curve. Fifth, moderate levels of PEEP were used in the helmet noninvasive ventilation group; the use of helmet noninvasive ventilation with higher levels of PEEP deserves further study.
Conclusions
Helmet noninvasive ventilation did not significantly reduce 28-day mortality compared with usual respiratory support among patients with acute hypoxemic respiratory failure due to COVID-19 pneumonia. However, interpretation of the findings is limited by imprecision in the effect estimate, which does not exclude potentially clinically important benefit or harm.
Trial Protocol and SAP
eTable 1. Characteristics of participating sites
eTable 2. Helmet noninvasive ventilation protocol
eTable 3. Inclusion and exclusion criteria
eTable 4. Primary outcome, secondary outcomes, and adverse events
eTable 5. Prespecified and post hoc subgroups
eTable 6. Patient enrollment by participating sites
eTable 7. Additional baseline characteristics
eTable 8. Additional information on interventions and co-interventions
eTable 9. Additional analyses on primary outcome
eTable 10. Additional information on secondary and adverse events
eTable 11. Summary of protocol violations
eFigure 1. Bedside poster for the initial settings of helmet noninvasive ventilation and general guidelines for intubation
eFigure 2. Dyspnea visual analog scale and device discomfort visual analog scales
eFigure 3. Kaplan-Meier time-to-event curves for 28-day mortality (Panel A) and intubation (Panel B) in the per-protocol population
eFigure 4. Serial respiratory rate, SaO2:FiO2, dyspnea visual analog scale, device discomfort visual analog scale, SOFA scores, and partial pressure of carbon dioxide (PaCO2)
eFigure 5. Prespecified subgroup analyses
eFigure 6. Post hoc subgroup analyses
eReferences
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Richardson S, Hirsch JS, Narasimhan M, et al. ; Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ute Muti-Schüenemann GE, Szczeklik W, Solo K, et al. Update alert 3: ventilation techniques and risk for transmission of coronavirus disease, including COVID-19. Ann Intern Med. 2022;175(1):W6-W7. doi: 10.7326/L21-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perkins GD, Ji C, Connolly BA, et al. ; RECOVERY-RS Collaborators . Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. 2022;327(6):546-558. doi: 10.1001/jama.2022.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reyes LF, Murthy S, Garcia-Gallo E, et al. ; ISARIC Clinical Characterisation Group . Clinical characteristics, risk factors and outcomes in patients with severe COVID-19 registered in the International Severe Acute Respiratory and Emerging Infection Consortium WHO clinical characterisation protocol: a prospective, multinational, multicentre, observational study. ERJ Open Res. 2022;8(1):00552-2021. doi: 10.1183/23120541.00552-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grieco DL, Maggiore SM, Roca O, et al. Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med. 2021;47(8):851-866. doi: 10.1007/s00134-021-06459-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avari H, Hiebert RJ, Ryzynski AA, et al. Quantitative assessment of viral dispersion associated with respiratory support devices in a simulated critical care environment. Am J Respir Crit Care Med. 2021;203(9):1112-1118. doi: 10.1164/rccm.202008-3070OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellani G, Grasselli G, Cecconi M, et al. Noninvasive ventilatory support of patients with COVID-19 outside the intensive care units (WARD-COVID). Ann Am Thorac Soc. 2021;18(6):1020-1026. doi: 10.1513/AnnalsATS.202008-1080OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. Coronavirus (COVID-19) update: daily roundup August 6, 2020.. Accessed April 25, 2022. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-august-6-2020
- 9.Chaudhuri D, Jinah R, Burns KEA, et al. Helmet noninvasive ventilation compared to facemask noninvasive ventilation and high-flow nasal cannula in acute respiratory failure: a systematic review and meta-analysis. Eur Respir J. 2022;59(3):2101269. doi: 10.1183/13993003.01269-2021 [DOI] [PubMed] [Google Scholar]
- 10.Ferreyro BL, Angriman F, Munshi L, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020;324(1):57-67. doi: 10.1001/jama.2020.9524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arabi YM, Tlayjeh H, Aldekhyl S, et al. Helmet non-invasive ventilation for COVID-19 patients (Helmet-COVID): study protocol for a multicentre randomised controlled trial. BMJ Open. 2021;11(8):e052169. doi: 10.1136/bmjopen-2021-052169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arabi Y, Aldekhyl S, Al Qahtani S, et al. Helmet noninvasive ventilation for COVID-19 patients (Helmet-COVID): statistical analysis plan for a randomized controlled trial. Trials. 2022;23(1):105. doi: 10.1186/s13063-021-05988-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi: 10.1001/jama.2016.6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grieco DL, Menga LS, Cesarano M, et al. ; COVID-ICU Gemelli Study Group . Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021;325(17):1731-1743. doi: 10.1001/jama.2021.4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grieco DL, Menga LS, Raggi V, et al. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2020;201(3):303-312. doi: 10.1164/rccm.201904-0841OC [DOI] [PubMed] [Google Scholar]
- 16.Hasan SS, Capstick T, Ahmed R, et al. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med. 2020;14(11):1149-1163. doi: 10.1080/17476348.2020.1804365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serafim RB, Póvoa P, Souza-Dantas V, Kalil AC, Salluh JIF. Clinical course and outcomes of critically ill patients with COVID-19 infection: a systematic review. Clin Microbiol Infect. 2021;27(1):47-54. doi: 10.1016/j.cmi.2020.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group . Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788-800. doi: 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 19.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 20.Winck JC, Scala R. Non-invasive respiratory support paths in hospitalized patients with COVID-19: proposal of an algorithm. Pulmonology. 2021;27(4):305-312. doi: 10.1016/j.pulmoe.2020.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVID-ICU Group, for the REVA Network, COVID-ICU Investigators . Benefits and risks of noninvasive oxygenation strategy in COVID-19: a multicenter, prospective cohort study (COVID-ICU) in 137 hospitals. Crit Care. 2021;25(1):421. doi: 10.1186/s13054-021-03784-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vargas F, Thille A, Lyazidi A, Campo FR, Brochard L. Helmet with specific settings versus facemask for noninvasive ventilation. Crit Care Med. 2009;37(6):1921-1928. doi: 10.1097/CCM.0b013e31819fff93 [DOI] [PubMed] [Google Scholar]
- 23.Nava S, Navalesi P. Helmet to deliver noninvasive ventilation: “handle with care”. Crit Care Med. 2009;37(6):2111-2113. doi: 10.1097/CCM.0b013e3181a5e6b5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and SAP
eTable 1. Characteristics of participating sites
eTable 2. Helmet noninvasive ventilation protocol
eTable 3. Inclusion and exclusion criteria
eTable 4. Primary outcome, secondary outcomes, and adverse events
eTable 5. Prespecified and post hoc subgroups
eTable 6. Patient enrollment by participating sites
eTable 7. Additional baseline characteristics
eTable 8. Additional information on interventions and co-interventions
eTable 9. Additional analyses on primary outcome
eTable 10. Additional information on secondary and adverse events
eTable 11. Summary of protocol violations
eFigure 1. Bedside poster for the initial settings of helmet noninvasive ventilation and general guidelines for intubation
eFigure 2. Dyspnea visual analog scale and device discomfort visual analog scales
eFigure 3. Kaplan-Meier time-to-event curves for 28-day mortality (Panel A) and intubation (Panel B) in the per-protocol population
eFigure 4. Serial respiratory rate, SaO2:FiO2, dyspnea visual analog scale, device discomfort visual analog scale, SOFA scores, and partial pressure of carbon dioxide (PaCO2)
eFigure 5. Prespecified subgroup analyses
eFigure 6. Post hoc subgroup analyses
eReferences
Nonauthor Collaborators
Data Sharing Statement