Benzylic oxidation of 2,3-dihydropyrrolo[1,2-a]indole at the 2α position.
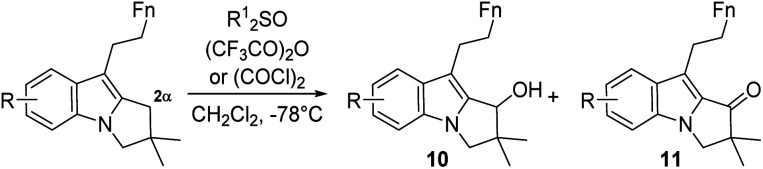
| ||||||
|---|---|---|---|---|---|---|
| entry | Substrate | Reagents (equiv.) | Base | Time(min) | Alcohol 10 (%) | Ketone 11 (%) |
| 1 | 6ca, R = H Fn = C4H9 | DPSO (3) TFAA (3) | — | 45 | 27 (10a) | — |
| 2 | " | DMSO (3) | — | 1 | Trace | 88 (11a) |
| TFAA (3) | ||||||
| 3 | 6a, b or 9 | " | — | 1 | Degradation | |
| 4 | 6b | DMSO (6) | NEt3 (3) | 60 | 64 (10b) | — |
| TFAA (3) | ||||||
| 5 | 7b | DMSO (6) | NEt3 (6) | 15 | 60 (10c) | 29 (11c) |
| (COCl)2 (3) | ||||||
| 6 | 9 | " | NEt3 (6) | 120 | No reaction | |
| 7 | " | " | — | 15 | — | 87 (11d) |
The model compound 6c was prepared according to the same gold catalysed strategy, see ESI. DPSO = diphenylsulfoxide. TFAA = trifluoroacetic anhydride.
