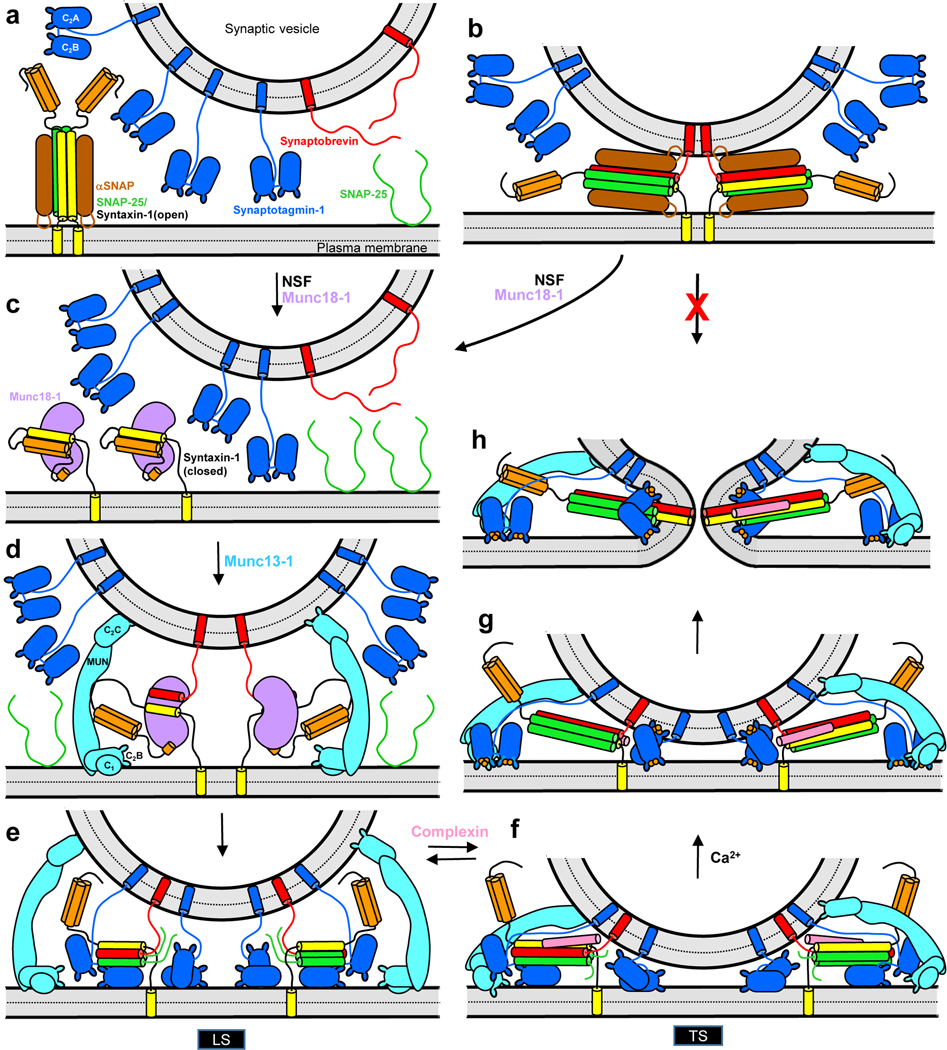
Figure 1.
Working model of the basic steps that lead to neurotransmitter release. (a) Diagram showing the localization of synaptobrevin (red) and synaptotagmin-1 (blue) on a synaptic vesicle, and of αSNAP (brown) bound to a 1:2 complex between SNAP-25 (green) and syntaxin-1 (SNARE motif yellow; Habc domain orange) on the plasma membrane. Helices formed by the SNAREs are represented by cylinders. αSNAP binding to the syntaxin-1-SNAP-25 complex hinders binding to synaptobrevin and SNARE complex formation. (b) Diagram illustrating that, even if trans-SNARE complexes between synaptobrevin, syntaxin-1 and SNAP-25 are formed, binding of αSNAP to these complexes prevents fusion, ensuring that neurotransmitter release does not occur through non-regulated pathways. (c) Diagram showing syntaxin-1 adopting a closed conformation that binds tightly to Munc18–1 (violet). This binary complex constitutes the starting point of the pathway that leads to neurotransmitter release. Closed syntaxin-1 may be available on the plasma membrane or may form after NSF dissociates an αSNAP-bound syntaxin-1-SNAP-25 complex (from panel a) or an αSNAP-bound trans-SNARE complex (from panel b). (d-e) The conserved C-terminal region of Munc13–1 (cyan) bridges the vesicle and plasma membranes through respective interactions involving the C2C domain and the C1-C2B region, and opens syntaxin-1 by binding to the linker between the syntaxin-1 Habc domain and SNARE motif. This action likely facilitates binding of Munc18–1 to synaptobrevin, forming a template complex (d) that initiates SNARE complex assembly upon binding of SNAP-25 to syntaxin-1 and synaptobrevin (e). Synaptotagmin-1 likely facilitates assembly by binding to SNAP-25 (not shown in d for simplicity). The synaptotagmin-1 C2B domain binds to the partially assembled SNARE complex through the primary interface and to the plasma membrane through the polybasic region (e). Munc13–1 facilitates SNARE complex assembly but also limits the number of SNARE complexes that form and hinders C-terminal zippering by bridging the two membranes in an approximately perpendicular orientation that characterizes a loose primed state (LS). This inhibitory action may be aided by formation of Munc13–1 clusters. (f) Further but not complete C-terminal zippering of the SNARE complex, which is favored by binding of complexin (pink), forces the two membranes closer together and Munc13–1 must bridge the membrane in a slanted orientation, forming a tight primed state (TS) that has a much higher probability of release upon Ca2+ influx than LS. Complexin and synaptotagmin-1 stabilize this state and prevent disassembly of the trans-SNARE complexes by NSF/αSNAP, but hinder final zippering to prevent premature fusion. (g-h) Ca2+ binding to synaptotagmin-1 causes dissociation from the SNARE complex, relieving the inhibition and thus allowing final C-terminal zippering and synaptic vesicle fusion. The dissociated synaptotagmin-1 molecules, or other synaptotagmin-1 molecules that were not bound to the SNAREs (shown in the middle) accelerate fusion through interactions with the lipids, perhaps because they perturb the bilayers, bridge the two membranes and/or induce membrane curvature. The increase in vesicular release probability caused by accumulation of Ca2+ and DAG during repetitive stimulation is proposed to arise because Ca2+ and DAG bind to the C2B and C1 domains of Munc13–1, respectively, and favor the slanted orientation, shifting the equilibrium from LS to TS. The following features are not shown for simplicity: (b, e-h) the linker region between the two SNAP-25 SNARE motifs, which remains anchored on the plasma membrane through plamitoylation; (f-h) the N- and C-terminal regions of complexin, which bind to the membranes; (e-h) Munc18–1, which may remain bound to the N-terminal region of syntaxin-1; (e-f) additional synaptotagmin-1 molecules that may bind to the SNARE complex through the tripartite interface or form a synaptotagmin-1 oligomeric ring that has been proposed to prevent fusion before Ca2+ influx and is dissociated upon Ca2+ binding.