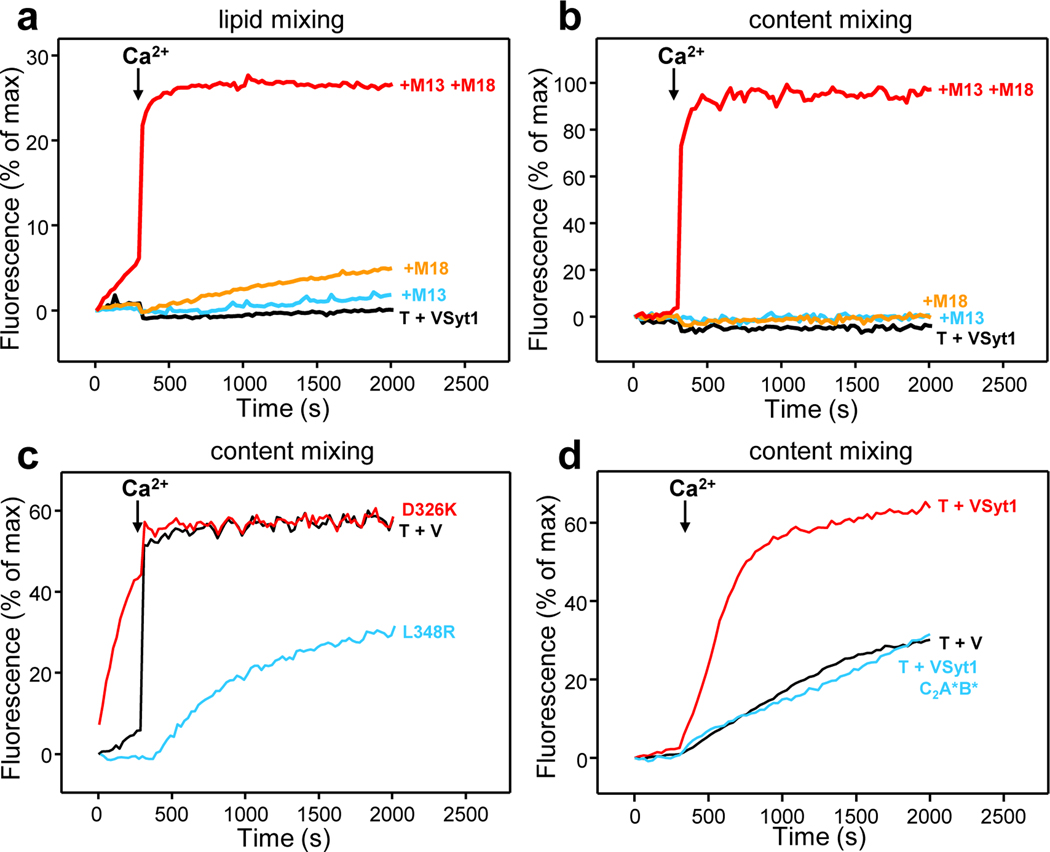
Figure 4.
Liposome fusion assays that recapitulate the dependence of synaptic vesicle fusion on Munc18–1, Munc13–1 and synaptotagmin-1. (a,b) Assays that monitored lipid mixing (a) and content mixing (b) of VSyt1-liposomes containing synaptobrevin (P:L ratio 1:500) and synaptotagmin-1 (P:L ratio 1:1,000) with T-liposomes containing syntaxin-1 (P:L ratio 1:800) bound to SNAP-25, in the presence of NSF and αSNAP. Under these conditions, there is no fusion without other additions (T + VSyt1, black curves). Inclusion of Munc18–1 and a Munc13–1 C-terminal fragment spanning the C1, C2B, MUN and C2C domains (C1C2BMUNC2C) leads to highly efficient Ca2+-dependent fusion (+M13 +M18, red curves), but there is no fusion when only one of these proteins is included (+M18, orange curves; +M13, blue curves) (85). Note that in the complete reaction with all the reagents there is some lipid mixing before Ca2+ addition, indicating that a few SNARE complexes are formed, but there is no content mixing, showing that there is not fusion. (c) Content mixing assays performed under analogous conditions but with V-liposomes containing only synaptobrevin (P:L ratio 1:500) in the presence of NSF, αSNAP, Munc13–1 C1C2BMUNC2C and WT Munc18–1 (T + V, black curve), Munc18–1 D326K (red curve) or Munc18–1 L348R (blue curve). The D326K mutation that unfurls the Munc18–1 loop leads to efficient fusion before Ca2+ addition, whereas the L348R mutation that impairs synaptobrevin binding to Munc18–1 strongly hinders fusion (140). Note that the Ca2+-dependent fusion observed with WT Munc18–1 is highly efficient even though synaptotagmin-1 was absent [compare black curve in panel (c) with red curve in panel (b)]. (d) Content mixing assays performed under analogous conditions but with VSyt1-liposomes containing synaptobrevin at 1:10,000 P:L ratio and synaptotagmin-1 at 1:1,000 ratio (T + VSyt1, red curve) or V-liposomes containing only synaptobrevin at 1:10,000 P:L ratio (T + V, black curve), in the presence of NSF, αSNAP, Munc13–1 C1C2BMUNC2C and WT Munc18–1. Note that inclusion of synaptotagmin-1 led to much more efficient fusion at this low synaptobrevin densities, and that experiments with VSyt1-lipsomes bearing synaptotagmin-1 with mutations in the Ca2+-binding sites of both C2 domains (T + VSyt1 C2A*B*, blue curve) led to similar fusion to that observed without synaptotagmin-1, showing that stimulation of fusion depends on Ca2+ binding to synaptagmin-1 (145).