Table 1. Optimization of Conditionsa.
| entry | ratio | solvent | catalyst | yieldb (%) |
|---|---|---|---|---|
| 1 | 1:1:1 | MeOH | Sc(OTf)3 | 24 |
| 2 | 2:1:2 | MeOH | Sc(OTf)3 | 74 |
| 3 | 2.2:1:2.2 | MeOH | Sc(OTf)3 | 64 |
| 4 | 1.3:1:1.3 | MeOH | Sc(OTf)3 | 34 |
| 5 | 2:1:2 | TFE | Sc(OTf)3 | 45 |
| 6 | 2:1:2 | water | Sc(OTf)3 | 11 |
| 7 | 2:1:2 | MeCN | Sc(OTf)3 | 36 |
| 8 | 2:1:2 | solvent free | Sc(OTf)3 | 21 |
| 9 | 2:1:2 | PEG40 | Sc(OTf)3 | 17 |
| 10 | 2:1:2 | MeOH | PTSA | 64 |
| 11 | 2:1:2 | MeOH | perchloric acid | 43 |
| 12 | 2:1:2 | MeOH | acetic acid | 48 |
| 13 | 2:1:2 | MeOH | NH4Cl | 51 |
| 14 | 2:1:2 | MeOH | ZrCl4 | 63 |
| 15c | 2:1:2 | MeOH | Sc(OTf)3 | 40 |
| 16d | 2:1:2 | MeOH | Sc(OTf)3 | 54 |
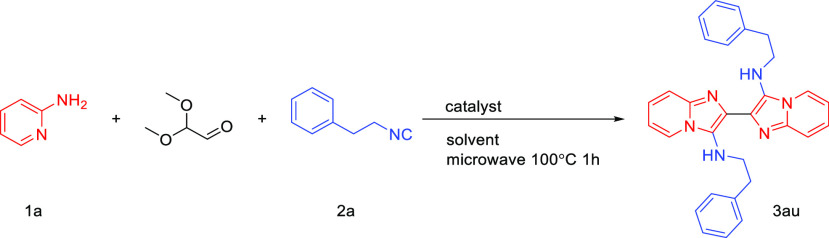
Reaction conditions: unless otherwise stated, all the reactions were performed with 1a (1 mmol), glyoxal dimethyl acetal (60% in H2O, 0.5 mmol), 2a (1 mmol), and catalyst (20 mol %) in solvent (0.5 mL) at 100 °C under microwave radiation for 1 h. PTSA = 4-methylbenzenesulfonic acid; TFE = trifluoroethanol.
Isolated yield.
Glyoxal (40 wt % in H2O) was used as dialdehyde source.
Heated at 80 °C for 12 h in sealed vial using aluminum heating blocks.