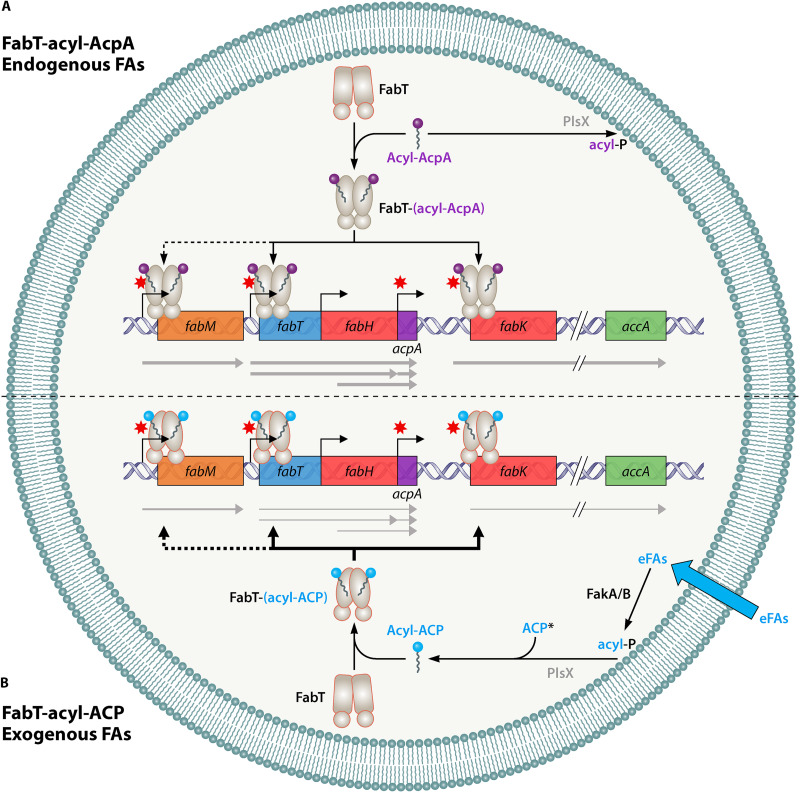
FIG 3.
Model of the FabT repression mechanism in S. pneumoniae and E. faecalis. (A) Endogenous fatty acid (FA) synthesis: an excess of FA synthesis generates acyl-AcpA, which binds to FabT; the complex binds with low affinity to the promoter regions of its target transcriptional units, leading to weak repression of FabT directly controlled genes, including acpA. Acyl-AcpA is also a substrate for the bidirectional glycerol-3-phosphate acyltransferase PlsX, leading to phospholipid synthesis (not shown). The minor apparent regulatory role of FabT when interacting with acyl-AcpA contrasts with its prominent role when exogenous FAs are present, e.g., as is common in vivo. (B) Exogenous FA utilization: entering FAs may be phosphorylated by fatty acid kinase complex FakA coupled to one of the FA-binding FakB subunits, each of which has FA preferences (22). Acyl-P is then converted to acyl-AcpB or AcpA (36) by a reverse PlsX reaction. Acyl-ACP binds to FabT, and the complex binds with high affinity to the promoter regions of its transcriptional unit targets, strongly repressing gene expression. The information contained in this figure corresponds to results shown in at least one species. Gray arrows under the genes represent the various transcriptional units described; their thickness symbolizes the level of transcription. Black arrows above or below the genes represent the binding of acyl-Acp to promoter regions; their thickness indicates repression efficiency; dotted arrows indicate that the repression level is species dependent. Note that only FabT-related functions are indicated. eFAs: exogenous FAs; acyl-P, acyl-phosphate. Note that acyl-AcpA is the FASII product; both acyl-AcpB and acyl-AcpA appear to be involved in eFA incorporation and reverse activity of PlsX.