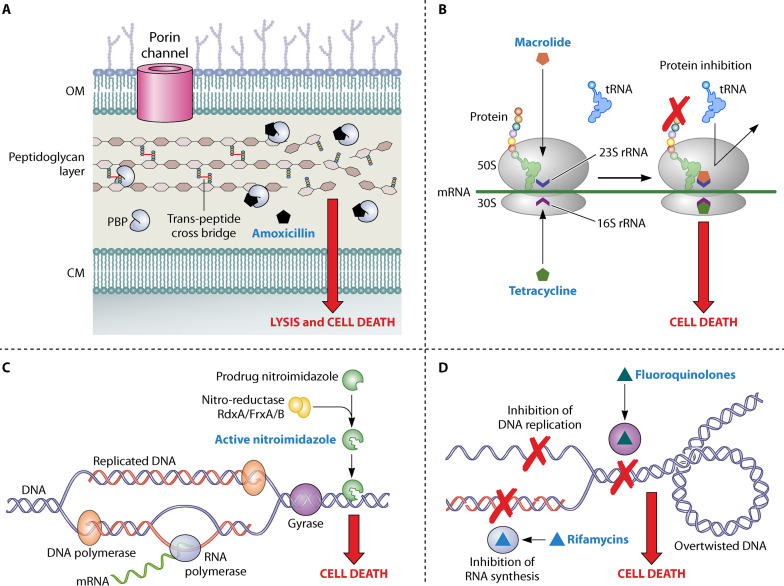
FIG 4.
Mechanism of antimicrobial activity in H. pylori. (A) During bacterial multiplication, the bacterial cell wall component (i.e., the multisheet peptidoglycan layer) is synthesized by penicillin-binding proteins (PBPs), which act as transpeptidases, causing the cross-linking of the peptidoglycan polymer chains. Beta-lactams (e.g., amoxicillin) binding with PBPs via penicillin binding motifs inhibit their action, preventing the synthesis of peptidoglycan layer and leading to bacterial cell lysis and cell death. (B) The bacterial ribosomes translate the mRNA to proteins. However, the macrolides (e.g., clarithromycin) and tetracyclines bind with 50S and 30S ribosomal subunits, respectively, inhibiting protein synthesis and causing bacterial cell death. (C) In the case of nitroimidazole (e.g., metronidazole), a prodrug is activated to its active form by reductases such as RdxA, FrxA, and FrxB. The activated metronidazole damages helicoidal DNA, causing bacterial cell death. (D) DNA replication and transcription of gene initiates with the formation of a replication fork, which creates supercoiled DNA with high tension in DNA strands. DNA gyrases (A and B) cause the unwinding of supercoiled DNA which is important for the normal DNA replication and transcription by RNA polymerases. Fluoroquinolones (e.g., levofloxacin) bind with DNA gyrases (A and B), whereas the rifamycins (e.g., rifabutin) bind with RNA polymerases and inhibit their respective functions, leading to bacterial cell death.