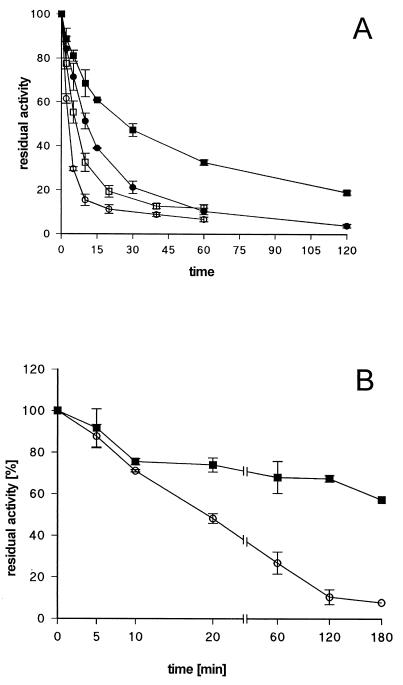
FIG. 4.
Stability of in vitro refolded wild-type and variant lipases. (A) Heat denaturation profiles. Cell lysates of E. coli BL21(DE3)(pLysS) expressing wild-type or C183S-C235S variant lipases were urea denatured and in vitro refolded. Samples were incubated for the indicated time periods at 50 and 60°C. The activity is expressed as residual activity relative to that of an untreated sample. ■, wild-type lipase at 50°C; ●, C183S-C235S lipase at 50°C; □, wild-type lipase at 60°C; ○, C183S-C235S lipase at 60°C. (B) Protease treatment. Cell lysates of E. coli BL21(DE3)(pLysS) expressing wild-type (■) or C183S-C235S variant lipases (○) were urea denatured and in vitro refolded. Samples were mixed with P. aeruginosa PABST7.1(pUCPKS) cell lysate at a ratio of 1:25 based on mass of protein. These mixtures were incubated at 37°C for the indicated time periods. Lipolytic activity is expressed as residual activity relative to that of an untreated sample. Activities were corrected for the activity determined for the P. aeruginosa cell lysate.