Abstract
Background and Objectives
The most common adverse events (AEs) after alemtuzumab (ALZ) include adverse infusion reactions, infections, and autoimmune disorders. Skin AEs are common during infusion, but there are few reported cases of long-term skin autoimmune disease.
Methods
A retrospective case series of patients developing long-term autoimmune skin disorders after ALZ administration in a tertiary care hospital.
Results
Of 133 patients treated with ALZ, 8 patients (6.02%) developed 9 autoimmune cutaneous AEs, including 4 events of alopecia areata, 2 of vitiligo, 2 of chronic urticaria, and 1 of inflammatory atrichia. Three of them occurred between the first and the second infusion.
Discussion
The lesions described are secondary to autoimmune disorders, probably related to immune dysregulation because of a differential lymphocyte repopulation after ALZ. Autoimmune cutaneous AEs may be frequent, and it would be recommended to monitor its appearance to treat them.

Alemtuzumab (ALZ) is an anti-CD52 monoclonal antibody approved for the treatment of relapsing-remitting multiple sclerosis.1 ALZ binds to CD52, causing cell lysis and depletion of T and B lymphocytes, followed by a differential lymphocyte repopulation.2 CD19 B-lymphocytes are repopulated during the first 3 to 6 months, reaching levels above normal at 12 months. CD8 T-lymphocytes take approximately 9–12 months to reach normal levels, whereas CD4 can take up to 1 or 2 years to reach normal levels.3,4
ALZ efficacy and safety has been evaluated in 3 pivotal studies: 1 phase II (CAMMS223) and 2 phase III (Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis 1 [CARE-MS] and 2 [CARE MS-II]). All of them were randomized and compared with an active comparator (interferon β-1a).5-7 The most commonly detected adverse events (AEs) detected with ALZ in such pivotal studies were adverse infusion reactions, increased risk of infections, and autoimmune disorders. The most common autoimmune disorders include thyroid events, autoimmune thrombocytopenia or other cytopenias (such as neutropenia or pancytopenia), and autoimmune nephropathies.5-11
These autoimmune disorders peak between 2 and 3 years after the administration of ALZ but may occur beyond 5 years after its infusion.3 The cause of secondary autoimmunity is still unknown, but different mechanisms have been proposed. One potential mechanism is differential lymphocyte repopulation, which can cause an imbalance in favor of B lymphocytes, favoring the production of autoantibodies in the absence of regulatory T cells.3,4 Another proposed mechanism involves T cell repopulation pathway, with homeostatic proliferation of cells escaping depletion predominating over thymic reconstitution—resulting in a less varied repertoire of T lymphocytes driven by IL-21—that has been linked to a higher risk of secondary autoimmunity.12,13
Other autoimmune disorders after ALZ, such as myositis,14 vasculitis,15 hemolytic anemia,16,17 or autoimmune hepatitis,18 are reported in the clinical literature. Skin AEs are common during infusion of the drug, and pruritic skin rash is very frequent.5-7 However, there are few published cases of long-term skin autoimmune diseases after ALZ. Recently, isolated cases of alopecia areata,19,20 vitiligo,21,22 and refractory chronic spontaneous urticaria23 have been reported.
The aim of this study is to define the incidence and evolution of autoimmune skin AEs in a sample of patients after receiving ALZ.
Methods
We present a single-center, retrospective case series of patients developing autoimmune skin disorders after ALZ administration in a MS care unit of a tertiary care hospital (MS care Unit, Virgen Macarena Hospital, Seville, Spain).
Patients have received ALZ between April 2009 and June 2020, and the follow-up has been performed in our MS unit. Data collection was conducted in 2020.
The diagnosis, treatment, and follow-up of the described autoimmune skin diseases have been performed by dermatology physicians.
We recorded gender, age of patients at the time of ALZ treatment, time since MS diagnosis, time since ALZ administration, the number of ALZ courses received, previous disease-modifying treatment, the type of autoimmune skin condition, and other concomitant autoimmune diseases.
Categorical variables are presented as frequencies and percentages.
Standard Protocol Approvals, Registrations, and Patient Consents
Received written informed consent was obtained from all patients (or guardians of patients) participating in the study (consent for research).
Data Availability
Anonymized data will be shared by request from any qualified investigator.
Results
Of 133 patients treated with ALZ, 8 patients (1 man and 7 women) developed long-term autoimmune cutaneous AEs (8.27%), with an average age of 34.88 and an average time since diagnosis of 14.75 years. One patient developed 2 different lesions, with an overall of 9 reported skin lesions. Three of them occurred between the first and second infusion; the other 6 occurred after the second course of treatment. No skin disorders were detected in consecutive cycles. Three patients (37.5%) switched from fingolimod as previous disease-modifying therapy (DMT) before ALZ, 3 (37.5%) from dimethyl fumarate, and 2 (25%) from natalizumab. Table shows a summary of the characteristics of these patients.
Table.
Patient Characteristics
Case 1
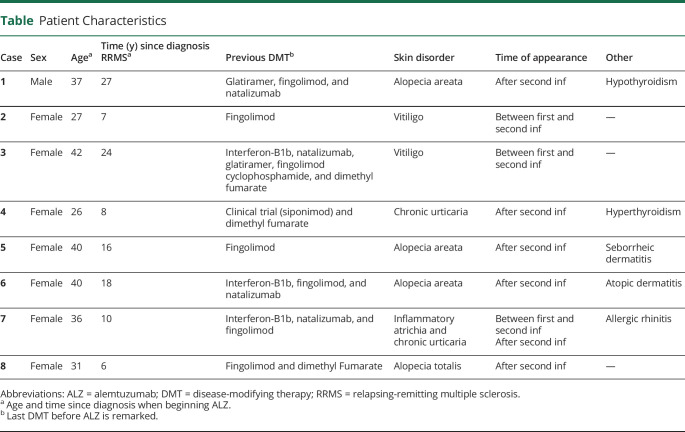
A man who received ALZ at the age 37 and after 27 years since diagnosis. Natalizumab was the previous DMT, stopped because of JC virus seroconversion. Thirteen months after the second infusion, he presented with hair loss on the bard and scalp and was diagnosed with alopecia areata (Figure 1). He started topical treatment with minoxidil, retinoic acid, and clobetasol and experienced clinical improvement. He also presented autoimmune hypothyroidism. He is currently stable and does not need additional ALZ cycles.
Figure 1. Alopecia Areata.
Case 2
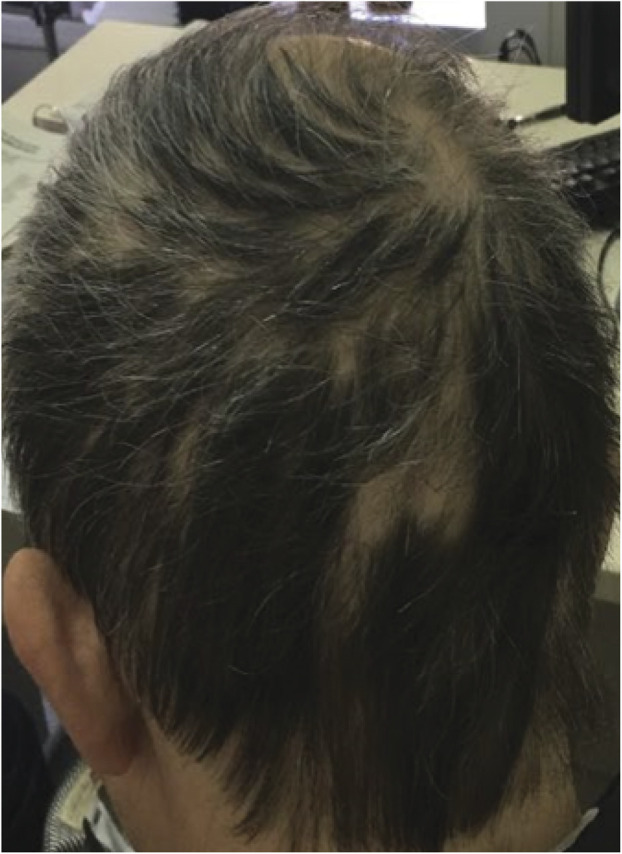
A woman who received ALZ at the age 27 and after 7 years since diagnosis after failure to fingolimod. Six months after the first infusion, she presented with hypopigmented macules on the limbs and genital area, appearing after a minor trauma. She was referred to dermatology and diagnosed with vitiligo with the Koebner phenomenon (Figure 2). Then, she began topical treatment with tacrolimus. After that, she received the second infusion. She is currently stable and does not need additional ALZ cycles.
Figure 2. Vitiligo With the Koebner Phenomenon.

Case 3
A woman who received ALZ at the age 42 and after 24 years since diagnosis after failure to dimethyl fumarate. She had failed several treatments before. Fifteen months after the first infusion, but before receiving the second cycle (it was delayed because of lymphopenia), she presented with hypopigmented macules and was diagnosed with vitiligo (Figure 3). Lesions remitted spontaneously without any treatment in a few months and did not show again after the second cycle. She needed no additional cycles.
Figure 3. Vitiligo.
Case 4
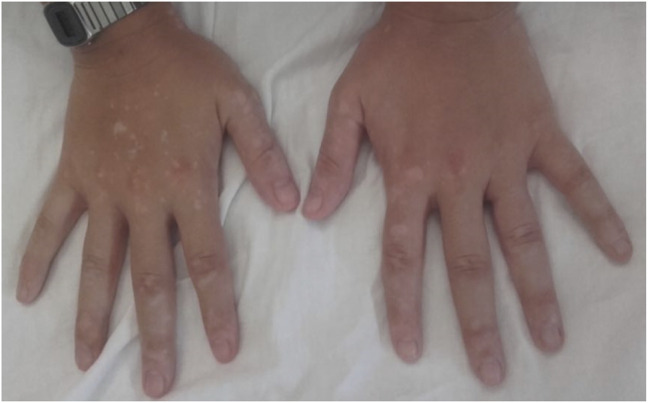
A woman who received ALZ at the age 26 and after 8 years since diagnosis after failure to dimethyl fumarate. Fourteen months after the second cycle, she presented with daily exanthematous and itchy lesions of less than 24 hours duration. She was referred to dermatology that described them as hives, and she was diagnosed with chronic spontaneous urticaria (Figure 4). Cyclosporine was proposed as the treatment from dermatology but was considered not convenient because of immunosuppression. She received several cycles of steroids, and nowadays, she maintains symptomatic treatment with antihistamines. She also presented Graves disease and Graves ophthalmopathy. She needed no additional cycles of ALZ.
Figure 4. Hives in a Patient With Chronic Urticaria.
Case 5
A woman who received ALZ at the age 40 and after 16 years since diagnosis after failure to fingolimod. Three months after the second cycle, she presented with hair loss located in her scalp and was diagnosed with alopecia areata (Figure 5). She is currently treated with topic methylprednisolone. After first infusion, she was diagnosed with seborrhoeic dermatitis too because of scalp peeling. She needed no additional cycles.
Figure 5. Alopecia Areata.
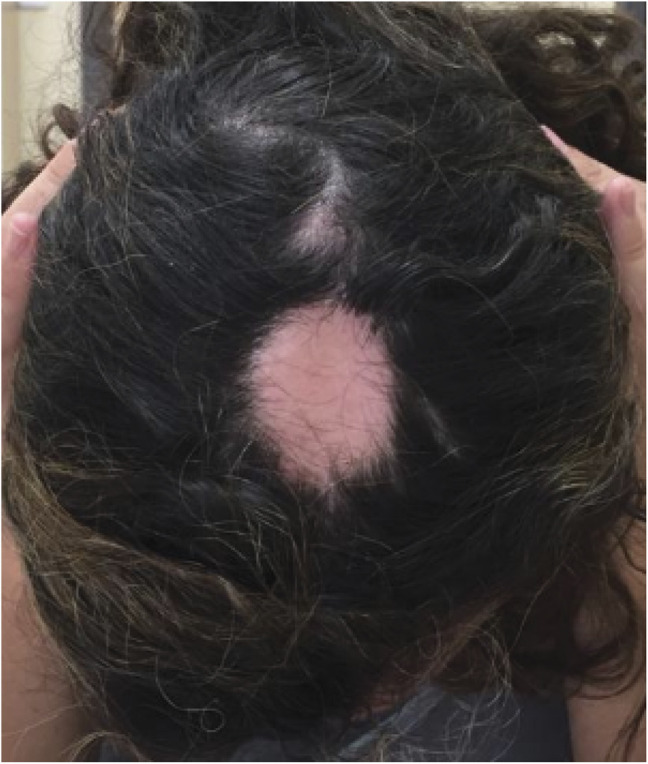
Case 6
A woman who received ALZ at the age of 40 and after 18 years since diagnosis. She had ceased natalizumab because of JC virus seroconversion. Twenty months after the second cycle, she presented with hair loss located in her scalp and was diagnosed with alopecia areata. She was treated with topic clobetasol and tacrolimus, and her evolution was favorable. She was then diagnosed with atopic dermatitis by dermatology because of peeling lesions on her back and arms. She needed no additional cycles.
Case 7
A woman who received ALZ at the age 36 and after 10 years since diagnosis after failure to fingolimod. A few months after the first infusion, she began to present with hair loss that was treated with biotin. Hair loss on the scalp improved, but she kept losing eyelash hair, with her condition worsening and presenting upper eyelid margin inflammation and pain after the second ALZ cycle. She was then referred to dermatology and diagnosed with inflammatory atrichia. In addition, 11 months after the second infusion, she began to present hives on her face of less than 24 hours duration and was diagnosed with chronic spontaneous urticaria that needed no treatment. She also presented allergic rhinitis as AE. She needed no additional cycles.
Case 8
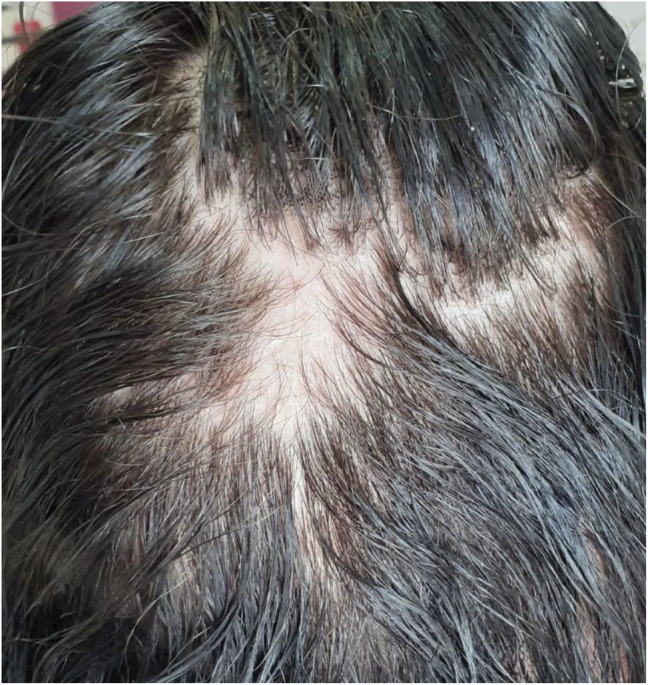
A woman who received ALZ at the age 31 and after 6 years since diagnosis after failure to dimethyl fumarate. Nine months after second infusion, she began to present with patchy hair loss in several areas, suggesting alopecia areata (Figure 6). Lesions converged until total hair loss on the scalp. She was referred to dermatology and was diagnosed with alopecia totalis. After treatment with corticosteroids infiltrated into the scalp, followed by oral and topical corticosteroids and minoxidil, the hair is starting to grow again. She needed no additional cycles.
Figure 6. Alopecia Areata Evolving to Alopecia Totalis.
Discussion
In our series, we have evidenced different long-term skin AEs after ALZ treatment. These cases share an autoimmune origin with cutaneous manifestations. As mentioned, cases of vitiligo21,22 and alopecia19,20 have been described before; a case of chronic spontaneous urticaria has been recently described,23 and dermatitis is also mentioned as an ALZ-related event.24
The pathophysiology of vitiligo has been widely described as an autoimmune phenomenon against skin melanocytes, leading to the appearance of depigmented areas. Alopecia areata is also determined by an autoimmune aggression around the hair follicle causing the growth to stop and its subsequent fall. We use the term alopecia totalis if it affects the whole scalp and alopecia universalis if it affects the body hair. CD8 T-lymphocytes are critically involved in these pathologies.21,25
Chronic spontaneous urticaria is determined by the appearance of wheals and/or angioedema for longer than 6 weeks. There are several theories of pathogenesis: the autoimmune origin is the most accepted hypothesis to explain inappropriate activation and degranulation of mast cells. Both a decrease in CD4 T-cells and an autoantibody-mediated disease process have been linked with this process and may explain its appearance after ALZ.23,26
ALZ is known to be associated with exanthems or dermatitis in the setting of therapy. These findings could be related to hypersensitivity reactions, but also to immune dysregulation.24 That could explain the long-term incidence of newly diagnosed dermatitis shown in our patients because 2 of our reported patients also presented with dermatitis. In addition, 1 patient treated with ALZ that has not been included in this work presented itchy lesions that were described as an unspecified dermatitis, but lesions vanished before a formal diagnosis could be established. We have not counted these lesions for the overall incidence because they have been heterogeneous, and the etiology is not clear because biopsies have not been performed.
Acne has been classically considered an infectious disease. However, it is an inflammatory process in which both innate immunity and propionibacterium acnes play an important role. It is related to other drugs that cause imbalance of innate immunity such as antiepidermal growth factor drugs.27 We have detected an increased incidence of acne within our patients treated with ALZ (3 patients without previous history of acne). This leads us to consider a possible immune dysregulation role. However, we have decided not to count the acne lesions in the overall of this work because we cannot rule out an infectious cause.
According to our data, alopecia areata and chronic urticaria tended to appear after the second infusion. As mentioned, 1 patient presented with lesions 15 months after the first infusion but before receiving the second cycle. Thus, lesions detected after the second cycle may be a late consequence of the first infusion. In addition, we found no new appearance of skin lesions after additional cycles following the second one.
As mentioned, isolated adverse effects have been previously reported, but this is the first series of cases encompassing all skin adverse effects. Thus, it allows us, for the first time, to infer the incidence of these effects.
The large cohort of patients treated with ALZ in our center allowed the detection of a wide variant of lesions in this study. Reported cases are supported by dermatology evaluations and photographic records.
Skin biopsies have not been performed, which, in some cases, prevents us from fully confirming the etiology of the lesions. In addition, cases are still too few to make assessments regarding the time of onset or incidence.
According to the overall prevalence (6.77%), autoimmune cutaneous AEs may be more frequent than other known autoimmune AE. Some of these findings may remain unnoticed if we are not vigilant to its appearance, so we recommend monitoring the appearance of cutaneous lesions that may be potentially treatable. Further studies to perform an accurate approach to the real prevalence of these injuries and its relation with autoimmunity and the drug are recommended.
Appendix. Authors
Contributor Information
Juan Luis Ruiz Peña, Email: jlruizpe@gmail.com.
M. Dolores Páramo Camino, Email: draparamo@gmail.com.
Juan Diego Guerra Hiraldo, Email: hiraldo.nrl@gmail.com.
Sara Eichau, Email: saraeichau@gmail.com.
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.European Medicines Agency. Alemtuzumab Datasheet. Accessed November 1, 2017, ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/003718/WC500150521.pdf [Google Scholar]
- 2.Hu Y, Turner MJ, Shields J, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology. 2009;128(2):260-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorensen PS, Sellebjerg F. Pulsed immune reconstitution therapy in multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419836913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menge T, Stüve O, Kieseier BC, Hartung HP. Alemtuzumab: the advantages and challenges of a novel therapy in MS. Neurology. 2014;83(1):87-97. [DOI] [PubMed] [Google Scholar]
- 5.Coles AJ, Compston DA, Selmaj KW, et al. ; CAMMS223 Trial Investigators. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786-1801. [DOI] [PubMed] [Google Scholar]
- 6.Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819-1828. [DOI] [PubMed] [Google Scholar]
- 7.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829-1839. [DOI] [PubMed] [Google Scholar]
- 8.Cuker A, Arnold DL, Cohen JA, et al. Detection and management of immune thrombocytopenia in alemtuzumab-treated patients in the multiple sclerosis clinical development program [poster]. Presented at the 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), 2015.
- 9.Cuker A, Coles AJ, Sullivan H, et al. A distinctive form of immune thrombocytopenia in a phase 2 study of alemtuzumab for the treatment of relapsing-remitting multiple sclerosis. Blood. 2011;118(24):6299-6305. [DOI] [PubMed] [Google Scholar]
- 10.Ghodasara RS, Rarick MB, Mosley MC, et al. Alemtuzumab causes significant, transient, post-infusion thrombocytopenia and other non-autoimmune cytopenias following initial and subsequent courses [poster]. Presented at the Consortium of Multiple Sclerosis Centers Annual Meeting, National Harbor, 2016.
- 11.Tuohy O, Costelloe L, Hill-Cawthorne G, et al. Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry. 2015;86(2):208-215. [DOI] [PubMed] [Google Scholar]
- 12.Jones JL, Thompson SA, Loh P, et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc Natl Acad Sci USA. 2013;110(50):20200-20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones JL, Phuah CL, Cox AL, et al. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J Clin Invest. 2009;119(7):2052-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aouad P, Yiannikas C, Fernando SL, Parratt J. A case of autoimmune myositis after treatment with alemtuzumab for multiple sclerosis. Mult Scler J Exp Transl Clin. 2018;4(4):2055217318819012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauer EM, Schliep S, Manger B, Lee DH, Linker RA. Microscopic polyangiitis after alemtuzumab treatment in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm. 2018;5(5):e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meunier B, Rico A, Seguier J, et al. Life-threatening autoimmune warm hemolytic anemia following treatment for multiple sclerosis with alemtuzumab. Mult Scler. 2018;24(6):811-813. [DOI] [PubMed] [Google Scholar]
- 17.Di ioia M, Farina D, Di tommaso V, et al. . Simultaneous early-onset severe autoimmune hemolytic anemia and albuminuria during alemtuzumab treatment for multiple sclerosis. Mult Scler. 2018;24(6):813-815. [DOI] [PubMed] [Google Scholar]
- 18.Carlson A, Bozinov N, Kipp L, Dunn J, Lock C. Autoimmune hepatitis during treatment of multiple sclerosis with alemtuzumab (P5.350). Neurology. 2018;90(15 suppl):1-15. [Google Scholar]
- 19.Alcalá C, Pzére-Miralles F, Gascón F, et al. Recurrent and universal alopecia areata following alemtuzumab treatment in multiple sclerosis: a secondary autoimmune disease. Mult Scler Relat Disord. 2019;27:406-408. [DOI] [PubMed] [Google Scholar]
- 20.Chan JK, Traboulsee AL, Sayao AL. Case of alemtuzumab-related alopecia areata management in MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(1):e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruck T, Pfeuffer S, Schulte-mecklenbeck A, et al. Vitiligo after alemtuzumab treatment: secondary autoimmunity is not all about B cells. Neurology. 2018;91(24):e2233-e2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eichau S, Lopez-ruiz R, Ruiz-pena JL, Paramo MD, Navarro-mascarell G, Izquierdo G. Vitiligo with Koebner phenomenon in a patient with multiple sclerosis treated with alemtuzumab. Rev Neurol. 2018;66(11):395-396. [PubMed] [Google Scholar]
- 23.Hu H, Reddell S, Riminton S, Chan C, Urriola N. Refractory chronic spontaneous urticaria after the use of alemtuzumab in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2020;7(2):e661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helm TN, Liu-Helm AY. Immunomodulation, alemtuzumab associated dermatitis and the histology of drug-induced exanthems. J Cutan Pathol. 2017;44(4):405-406. [DOI] [PubMed] [Google Scholar]
- 25.Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78(1):1-12. [DOI] [PubMed] [Google Scholar]
- 26.Jain S. Pathogenesis of chronic urticaria: an overview. Dermatol Res Pract. 2014;2014(8):674709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piérard-Franchimont C, Blaise G, Paquet P, Quatresooz R, Rorive A, Piérard GE. Paroxysmal iatrogenic acne and the epidermal growth factor receptor inhibitors (EGFR). Rev Med Liege. 2007;62(1):11-14. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.