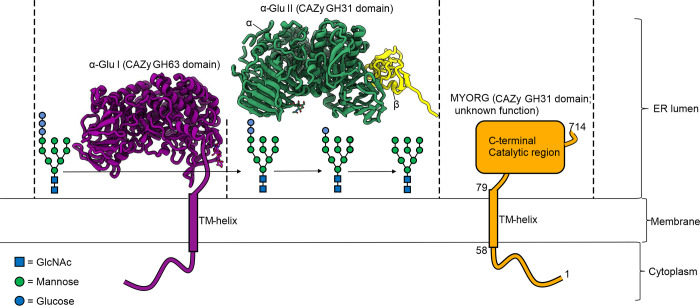
Fig 1. ER lumen α-glycosidases play roles in N-glycan processing.
Crystal structures of Mus musculus α-glucosidase I (PDB: 5MHF) and II (PDB: 5F0E). The transfer of Glc3Man9GlcNAc2 onto nascent polypeptide chains initiates an ER localised quality control process wherein the terminal nonreducing α1-2-linked glucose and the 2 inner α1-3-linked glucose residues are hydrolysed by α-Glu I and α-Glu II, respectively. Retention in the ER of glycoproteins bearing the innermost α1-3-linked glucose by the chaperones calnexin and calreticulin coupled with re-attachment of α1-3-linked glucose to misfolded proteins by UDP-glucose:glycoprotein glucosyltransferase regulates protein quality control. The function of MYORG within the ER and relevance to glycoprotein processing is unknown. The symbols used for monosaccharides follow the recommendations of the CFG. CFG, Consortium for Functional Glycomics; ER, endoplasmic reticulum; MYORG, myogenesis-regulating glycosidase.