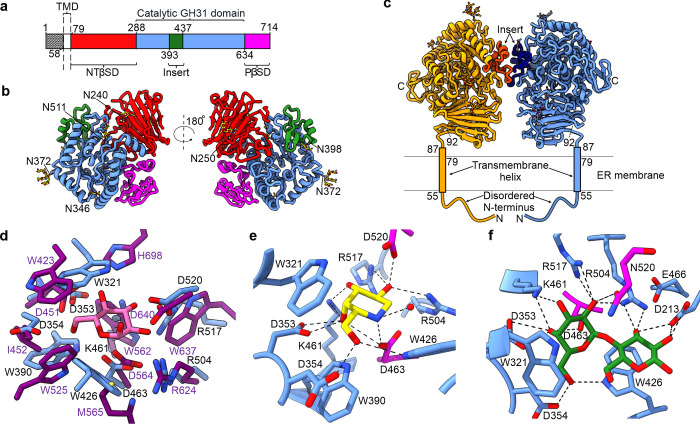
Fig 3. MYORG is a membrane bound dimer that selectively binds an unusual Gal-α1,4-Glc epitope.
(a) Domain boundaries of MYORG with numbering representing the last residue of the domain. (b) Cartoon ribbon representation of MYORG with N-glycans depicted as sticks with the glycosylated Asn residues labelled. (c) The MYORG dimer arrangement showing the insert region and the expected orientation of MYORG with respect to the ER membrane based on analyses using PSIPRED [27], DISOPRED [28], and MEMSAT [29]. (d) Comparison of the active site of MYORG (blue, residue labelling in black) with that of Mmα-Glu-II (purple; PDB: 5H9O). D-glucose is bound by Mmα-Glu-II and is depicted in pink. (e) Residues involved in the positioning and binding of DGJ. (f) Residues involved in binding Gal-α1,4-Glc. Dashed lines in (e) and (f) represent hydrogen bonding. Magenta sticks are used to emphasise the catalytic acid (D520, mutated to N520 in (f)) and nucleophile (D463) residues. DGJ, deoxygalactonojirimycin; ER, endoplasmic reticulum; MYORG, myogenesis-regulating glycosidase; NTβSD, N-terminal β-sheet domain; PβSD, proximal β-sheet domain; TMD, transmembrane domain.