Abstract
The efficacy of novel oral anticoagulants (NOACs) in severely obese patients is uncertain as volume of distribution is related to weight, and few such patients were enrolled in the pivotal trials. As the month after direct-current cardioversion (DCCV) for atrial fibrillation and atrial flutter is a high-risk period for stroke, we sought to evaluate the safety of performing DCCV in obese patients on NOAC. All patients who underwent DCCV after ≥3 weeks of NOAC or therapeutic warfarin treatment without a previous transesophageal echocardiogram over a 3-year period at a single center were included. Obesity groups were defined as normal (body mass index [BMI] < 25), overweight (BMI 25 to <30), class 1 obesity (BMI 30 to <35), class 2 obesity (BMI 35 to <40), and class 3 or severe obesity (BMI ≥ 40). The primary end point was stroke at 30 days. Of 761 patients, 73 were severely obese, 78 class 2 obese, 197 class 1 obese, 254 overweight, and 159 in the normal weight group. Average age 66.4 ± 10.3 years and 32.5% women. Mean CHA2DS2-VASc score was 2.6 ± 1.6, and 78.9% were on NOACs with no differences in groups. There were no strokes in the severely obese group, and 1 each in class 2 obesity and normal weight (p = 0.3). In conclusion, there was a low rate of stroke in all weight classes after DCCV in patients taking NOACs and warfarin. NOAC use in severely obese patients who underwent DCCV appears safe even in the absence of transesophageal echocardiogram.
Novel oral anticoagulants (NOACs) have become widely adopted in patients with nonvalvular atrial fibrillation (AF).1 Variations in the volume of distribution of these fixed-dose drug regimens at extremes of body weight raise some pharmacokinetic concerns, particularly the potential for lower drug concentrations and shorter half-lives, resulting in under-dosing.2 However, few patients at very elevated weights were included in the original NOAC trials for either nonvalvular AF or prevention of venous thromboembolism.3-5 Current cardiology guidelines do not discuss changes in use of NOACs in obese patients, whereas the hematology guidelines recommend against the use of NOACs in patients with body mass index (BMI) >40 or weight >120 kg, as well as checking drug-specific serum levels, but evidence for this strategy remains limited.2,6 Electrical cardioversion (direct-current cardioversion [DCCV]) is associated with an elevated risk of stroke or thromboembolism within the first 30 days due to the potential for dislodging a thrombus formed in the static left atrium or left atrial appendage, or formation of a thrombus as a result of left atrial stunning after restoration of sinus rhythm.7,8 This risk of thromboembolism is mitigated either by ensuring 3 to 4 weeks of therapeutic anticoagulation before DCCV or by utilizing transesophageal echocardiography (TEE) to evaluate for left atrial or left atrial appendage thrombus. The purpose of this study is to evaluate the safety of NOACs for stroke prevention in AF in obese patients in the peri-DCCV period without previous TEE.
Methods
A retrospective analysis was performed on 1386 elective DCCVs done at a single medical center (Northwestern Memorial Hospital) from January 2015 to September 2017. Of these, 761 were performed without TEE guidance and included in the present study. All 761 patients had at least 3 weeks of NOAC or therapeutic warfarin treatment before DCCV. Decisions regarding selection of anticoagulant were at the discretion of the treating physician.
The DCCVs were performed in a noninvasive procedure room by either a credentialed advanced practice provider or cardiology fellow under the supervision of an attending electrophysiologist.9 After a standard history and physical and informed consent, Quik-Combo pads were applied to the patient in the anterior-posterior orientation and connected to a biphasic defibrillator. An anesthesiologist provided Monitored Anesthesia Care with deep sedation for each procedure. Synchronized cardioversion was performed with initial shock at 200 J for AF, 100 J for atrial flutter, and 50 J for atrial tachycardia. In the case of immediate return of AF, a second shock was performed, with increasing energy (up to 360 J for AF). Subsequent shocks or use of ibutilide was at the discretion of the attending electrophysiologist.
Obesity groups followed the definitions from the Centers for Disease Control and Prevention and were classified as normal (BMI < 25), overweight (BMI 25 to <30), class 1 obesity (BMI 30 to <35), class 2 obesity (BMI 35 to <40), and class 3 or severe obesity (BMI ≥ 40).10 The primary end point was stroke at 30 days. Additional analysis was done by weight class, defined simply as ≥ or <120 kg.
Continuous variables were expressed as mean ± standard deviation. A 1-way analysis of variance was used to compare >2 means, and student’s t test was used to compare 2 continuous variables. Chi-square test was used to compare proportions. A p value of <0.05 was considered significant. In the case of non-normally distributed variables, nonparametric adjustment was done.
Results
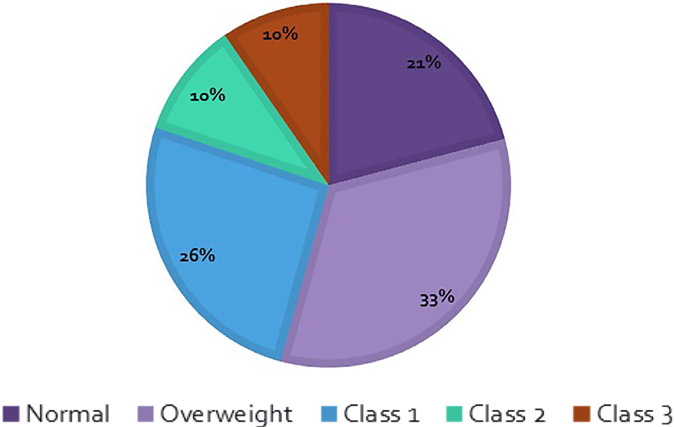
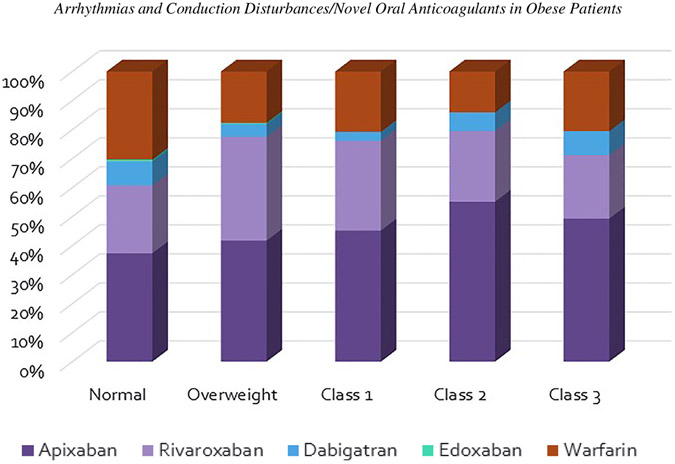
Of 761 patients included in this study, 159 were classified as normal by BMI, 254 in the overweight group, 197 patients in the class 1 obese group, 78 in class 2, and 73 in class 3 or severe obesity (Figure 1). There were just 3 patients who would be considered underweight by BMI and they were included with the normal group. Baseline characteristics are shown in Table 1. Of note, the obese patients were significantly younger, but without difference in CHA2DS2-VASc score (overall average 2.6 ± 1.6). There was no significant difference in individual anticoagulant choice by BMI class (Figure 2). Apixaban was the most commonly used anticoagulant in each class, followed by rivaroxaban. Together, apixaban and rivaroxaban accounted for 92.8% of the NOAC usage overall. When groups are separated by absolute weight (≥ or <120 kg), the higher weight group was more likely to be younger, male, have a lower CHA2DS2-VASc score, and a larger left atrium. There was no significant difference in the usage of each NOAC between the 2 weight groups (Table 2).
Figure 1.
Distribution of BMI classifications.
Table 1.
Baseline characteristics by body mass index (kg/m2)
| Variable | < 25 | 25 – < 30 | 30 – < 35 | 35 – < 40 | ≥ 40 | p value |
|---|---|---|---|---|---|---|
| Age (Years) | 71.6 ± 11.8 | 68.7 ± 11.0 | 67.2 ± 9.9 | 66.7 ± 9.2 | 62.0 ± 10.8 | <0.01 |
| Women | 73 (45.9%) | 53 (20.9%) | 73 (37.2%) | 23 (29.4%) | 24 (32.9%) | 0.03 |
| Weight (kg) | 67.9 ± 11.7 | 86.9 ± 11.7 | 98.5 ± 13.9 | 114 ± 16.0 | 131 ± 20.3 | <0.01 |
| CHA2DS2-VASc score | 3.0 ± 1.7 | 2.5 ± 1.6 | 2.6 ± 1.7 | 2.7 ± 1.6 | 2.4 ± 1.4 | 0.04 |
| Left ventricular ejection fraction (%) | 53.1 ± 11.6 | 54.9 ± 10.8 | 54.0 ± 11.9 | 55.1 ± 10.2 | 52.4 ± 12.5 | 0.38 |
| Left atrial diameter (cm) | 4.2 ± 0.7 | 4.2 ± 0.7 | 4.3 ± 0.6 | 4.4 ± 0.7 | 4.5 ± 0.9 | 0.05 |
| Presenting rhythm | ||||||
| - Atrial fibrillation | 105 (66.0%) | 203 (79.9%) | 161 (81.7%) | 72 (92.3%) | 69 (94.5%) | 0.18 |
| - Atrial flutter | 51 (32.1%) | 47 (18.5%) | 35 (17.8%) | 6 (7.7%) | 3 (4.1%) | <0.01 |
| - Atrial tachycardia | 3 (1.9%) | 4 (1.6%) | 1 (0.5%) | 0 (0%) | 1 (1.4%) | 0.19 |
| Antiarrhythmic drug use | ||||||
| - Any antiarrhythmic | 99 (62.3%) | 164 (64.5%) | 121 (61.4%) | 57 (73.1%) | 48 (65.7%) | 0.86 |
| - Amiodarone | 32 (20.1%) | 56 (22.0%) | 45 (22.8%) | 31 (39.7%) | 20 (27.4%) | 0.05 |
| - Dofetilide | 12 (7.5%) | 23 (9.0%) | 12 (6.1%) | 7 (9.0%) | 7 (9.6%) | 0.92 |
| - Dronedarone | 18 (11.3%) | 28 (11.0%) | 23 (11.7%) | 5 (6.4%) | 12 (16.4%) | 0.33 |
| - Flecainide | 10 (6.3%) | 20 (7.9%) | 16 (8.1%) | 4 (5.1%) | 0 (0%) | 0.08 |
| - Propafenone | 18 (11.3%) | 19 (7.5%) | 11 (5.6%) | 4 (5.1%) | 2 (4.1%) | 0.27 |
| - Sotalol | 9 (5.7%) | 18 (7.1%) | 14 (7.1%) | 6 (7.7%) | 7 (9.6%) | 0.86 |
| Anticoagulation | ||||||
| - Warfarin | 48 (30.2%) | 45 (17.7%) | 41 (20.8%) | 11 (14.1%) | 15 (20.5%) | 0.13 |
| - Apixaban | 59 (37.1%) | 106 (41.7%) | 89 (45.2%) | 43 (55.1%) | 36 (49.3%) | 0.38 |
| - Rivaroxaban | 37 (23.3%) | 91 (35.8%) | 61 (31.0%) | 19 (24.4%) | 16 (21.9%) | 0.26 |
| - Dabigatran | 13 (8.2%) | 11 (4.3%) | 6 (3.0%) | 5 (6.4%) | 6 (8.2%) | 0.43 |
| - Edoxaban | 1 (0.6%) | 1 (0.4%) | 0 (0%) | 0 (0%) | 0 (0%) |
Figure 2.
Anticoagulant usage by obesity class.
Table 2.
Baseline characteristics by absolute body weight (kg)
| Variable | <120 kg (n = 660) |
≥120 kg (n = 101) |
p value |
|---|---|---|---|
| Age (Years) | 68.9 ± 11.0 | 62.4 ± 9.0 | <0.01 |
| Women | 219 (33.2%) | 18 (17.8%) | <0.01 |
| Body mass index (kg/m2) | 28.7 ± 5.5 | 40.9 ± 6.1 | <0.01 |
| Weight (kg) | 86.7 ± 16.7 | 134 ± 14.7 | <0.01 |
| CHA2DS2-VASc score | 2.7 ± 1.7 | 2.2 ± 1.4 | <0.01 |
| Left ventricular ejection fraction (%) | 54.3 ± 11.1 | 52.2 ± 12.9 | 0.08 |
| Left atrial diameter (cm) | 4.2 ± 0.7 | 4.6 ± 0.8 | <0.01 |
| Presenting rhythm | |||
| - Atrial fibrillation | 516 (78.2%) | 94 (93.1%) | <0.01 |
| - Atrial flutter | 136 (20.6%) | 6 (5.9%) | <0.01 |
| - Atrial tachycardia | 8 (1.2%) | 1 (1.0%) | 0.86 |
| Antiarrhythmic drug use | |||
| - Any antiarrhythmic | 423 (64.1%) | 59 (58.4%) | 0.27 |
| - Amiodarone | 152 (23.0%) | 32 (31.7%) | 0.06 |
| - Dofetilide | 55 (8.3%) | 6 (5.9%) | 0.41 |
| - Dronedarone | 73 (11.1%) | 6 (5.9%) | 0.11 |
| - Flecainide | 46 (7.0%) | 4 (4.0%) | 0.26 |
| - Propafenone | 51 (7.7%) | 3 (3.0%) | 0.09 |
| - Sotalol | 46 (7.0%) | 8 (7.9%) | 0.74 |
| Anticoagulation | |||
| - Warfarin | 142 (21.5%) | 18 (17.8%) | 0.39 |
| - Apixaban | 287 (43.5%) | 46 (45.5%) | 0.71 |
| - Rivaroxaban | 192 (29.1%) | 32 (31.7%) | 0.59 |
| - Dabigatran | 36 (5.5%) | 5 (5.0%) | 0.84 |
| - Edoxaban | 2 (0.3%) | 0 (0%) | 0.58 |
Two patients (0.26%) in the study had cardioembolic strokes after DCCV. One patient had a BMI of 38 (class 2 obesity) with a total weight of 100.6 kg; the other patient had a BMI of 22.5 (normal) with a total weight of 62 kg. Both patients had been taking apixaban before DCCV. Other adverse events were 2 episodes of bradycardia requiring medication intervention in the class 2 obesity group.
Discussion
In our study of 761 patients on NOACs who underwent DCCV without previous TEE, there was a very low rate of stroke in all weight classes and none in patients with BMI ≥40. These data suggest that NOAC use for stroke prevention in nonvalvular AF even in severely obese patients appears safe. The stroke rate of 0.26% is similar to that found in previous studies of DCCV in AF. Klein et al found a rate of 0.5% in patients who had undergone a previous TEE and 0.8% in those who had not had a TEE but rather received 3 weeks of therapeutic anticoagulation with warfarin.11 Weight and BMI were not specified in these previous studies. Other studies have found a 0.0% rate of stroke post-DCCV with anticoagulation and up to 1.3% without previous anticoagulation.7,8 Both patients in our study who have strokes were diagnosed the day after DCCV. In prior published studies, there is a wide range of when the stroke occurred, from day 0 to day 55 and it is likely that the finding of the occurrence of the 2 strokes on day 1 is merely a coincidence.7,11
Few patients of elevated weight were included in the original trials of NOACs for nonvalvular AF. In the RE-LY trial of dabigatran, there were 3,099 patients with weights over 100 kg, and a subgroup analysis of patients with BMI <28 compared with ≥28 did not show any significant difference in efficacy (p = 0.21).3 In ARISTOTLE, the apixaban trial in nonvalvular AF, median weight was 82 kg with an interquartile range of 70 to 96 kg. Subgroup analysis by weight evaluated difference with low body weight but not high (comparing only <60 kg vs ≥60 kg).4 The rivaroxaban AF trial, ROCKET-AF, reported a median BMI of 28.3 (interquartile range of 25 to 32). Subgroup analysis by BMI of ≤25, 25 to 35, and >35 showed no difference in efficacy in those groups (p = 0.54). Of note, the 972 patients on rivaroxaban with BMI >35 represented 13.6% of patients in the trial.5 Consequently, generalization of those findings to the obese population is limited, and we are aware of no previous descriptions of the use of NOACs in morbidly obese patients who underwent DCCV.
Fixed dosing of NOACs as compared with weight-adjusted dosing for warfarin and heparin products has raised concerns for insufficiently low therapeutic levels of the anticoagulant in the plasma for obese patients. However, pharmacokinetic and pharmacodynamics studies in healthy subjects have demonstrated only smaller variations, believe not to be clinically significant. A study of rivaroxaban in healthy volunteers of weight over 120 kg compared with normal weight showed that differences in inhibition of factor Xa activity were <10%.12 Another study in rivaroxaban developed a pharmacokinetic model which correlated plasma drug levels with body weight, suggesting higher levels of rivaroxaban in more obese patients, but unclear how this would affect clinical anticoagulation.13
In a phase I study of apixaban in healthy subjects of different weights, patients with weight >120 kg has 20% lower apixaban exposure, but the investigators of that study believe this effect to be modest and unlikely to be of clinical significance. In the high weight group, 19 patients were included, with a mean weight of 137 kg (interquartile range 120 to 175) and mean BMI 42.6 (interquartile range 32 to 54). This study required that patients in the high weight group have a BMI of at least 30 to more accurately reflect an obese population. It should be noted, however, that this study used a dose of 10 mg of apixaban, which is higher than the standard dose for stroke prevention in nonvalvular AF.14
The NOACs have also been studied extensively for prevention of venous thromboembolism; in a meta-analysis of the 6 major phase III clinical trials of the NOACs for prevention of venous thromboembolism, there was similar efficacy between those weighing < 100 kg and those weighing ≥100 kg when compared with standard therapeutic warfarin.15
The limitations of this study included the nonrandomized nature of a cohort study. Additionally, patients with previous TEE were excluded, so it is possible that a planned DCCV could have been cancelled due to identifying a thrombus during the TEE. Furthermore, given the very low rate of stroke, this study was underpowered to detect differences in overall stroke rates.
In conclusion, there was a low rate of stroke in all weight classes after DCCV in patients taking NOACs and warfarin. NOAC use in severely obese patients who underwent DCCV appears safe even in the absence of TEE. Further investigation with randomized trials involving higher percentages of obese patients are needed.
Footnotes
Disclosures
None.
References
- 1.Steinberg BA, Gao H, Shrader P, Pieper K, Thomas L, Camm AJ, Ezekowitz MD, Fonarow GC, Gersh BJ, Goldhaber S, Haas S, Hacke W, Kowey PR, Ansell J, Mahaffey KW, Naccarelli G, Reiffel JA, Turpie A, Verheugt F, Piccini JP, Kakkar A, Peterson ED, Fox KAA, Garfield AF. Investigators O-A. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J 2017;194:132–140. [DOI] [PubMed] [Google Scholar]
- 2.Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost 2016;14:1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, Committee R-LS. Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L, Committees A. Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM, Investigators RA. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 6.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr., Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. American College of Cardiology/American Heart Association Task Force on Practice G. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 7.Gentile F, Elhendy A, Khandheria BK, Seward JB, Lohse CM, Shen WK, Bailey KR, Montgomery SC, Burger KN, Tajik AJ. Safety of electrical cardioversion in patients with atrial fibrillation. Mayo Clin Proc 2002;77:897–904. [DOI] [PubMed] [Google Scholar]
- 8.Arnold AZ, Mick MJ, Mazurek RP, Loop FD, Trohman RG. Role of prophylactic anticoagulation for direct current cardioversion in patients with atrial fibrillation or atrial flutter. J Am Coll Cardiol 1992;19:851–855. [DOI] [PubMed] [Google Scholar]
- 9.Strzelczyk T, Kaplan RM, Medler M, Knight BP. Outcomes associated with electrical cardioversion for atrial fibrillation when performed autonomously by an advanced practice provider. JACC Clin Electrophysiol 2017;3:1447–1452. [DOI] [PubMed] [Google Scholar]
- 10.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG, Investigators P. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N EnglJMed 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 11.Klein AL, Grimm RA, Murray RD, Apperson-Hansen C, Asinger RW, Black IW, Davidoff R, Erbel R, Halperin JL, Orsinelli DA, Porter TR, Stoddard MF. Assessment of cardioversion using transesophageal echocardiography I. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med 2001;344:1411–1420. [DOI] [PubMed] [Google Scholar]
- 12.Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. J Clin Pharmacol 2007;47:218–226. [DOI] [PubMed] [Google Scholar]
- 13.Mueck W, Lensing AW, Agnelli G, Decousus H, Prandoni P, Misselwitz F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet 2011;50:675–686. [DOI] [PubMed] [Google Scholar]
- 14.Upreti VV, Wang J, Barrett YC, Byon W, Boyd RA, Pursley J, LaCreta FP, Frost CE. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol 2013;76:908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 2014;124:1968–1975. [DOI] [PubMed] [Google Scholar]