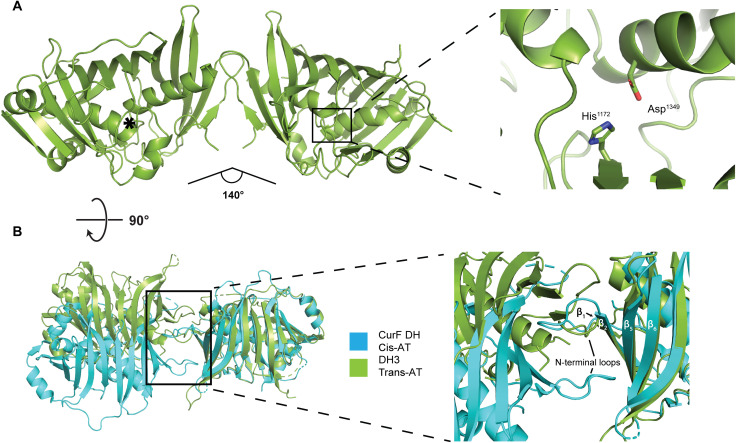
Fig. 3. The dimeric structure of the isolated DH3 domain of K3DAK4.
(A) Overview of the 2.2-Å resolution crystal structure of DH3 (left) and a close-up view of one active site (right). The active site of the second protomer is indicated by an asterisk. The angle between the two protomers is indicated. (B) Comparison of the dimer interface of DH3 (green) with the dehydratase domain of the cis-AT PKS CurF (turquoise). Models are aligned on the right protomer. The inset highlights the different topology of β strand and N-terminal loop interactions in the dimerization interfaces of DH3 and CurF DH.