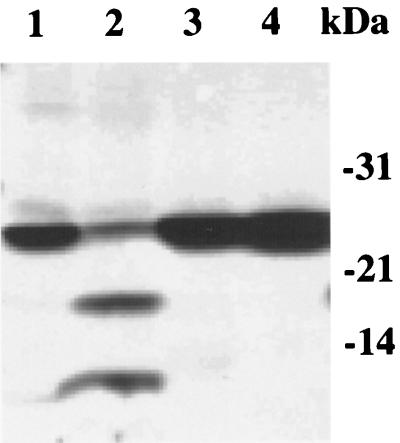
FIG. 5.
SDS-PAGE analysis of in vitro self-cleavage catalyzed by S. pneumoniae signal peptidase I. Reactions (20 μl) containing 2 to 4 μg of wild-type signal peptidase I or its mutants were incubated at 37°C for 1 h in 20 mM Tris-HCl–0.05% Triton X-100–5% glycerol–100 μg of E. coli lipid extract. The samples were separated by 4 to 20% gradient SDS-PAGE, and the gel was stained with Coomassie brilliant blue. Lane 1, purified wild-type signal peptidase I before incubation; lane 2, wild-type signal peptidase I after incubation; lane 3, mutant S38A after incubation; lane 4, mutant K76A after incubation.