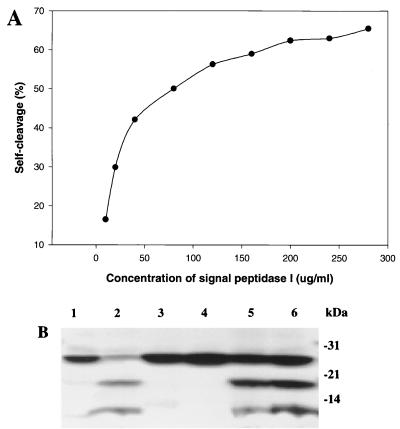
FIG. 6.
Self-cleavage of S. pneumoniae signal peptidase I is an intermolecular process. (A) Self-cleavage activity of signal peptidase I is dependent on protein concentration. Reaction mixtures (20 μl) containing different concentrations of signal peptidase I as indicated were incubated at 37°C for 30 min. The samples were separated on a 4 to 20% gradient SDS-polyacrylamide gel, and the gel was stained with Coomassie brilliant blue. Densitometer analysis was performed with a Personal Densitometer SI and Image Quant 5.0 software from Molecular Dynamics. (B) Signal peptidase I mutants S38A and K76A were cleaved by wild-type signal peptidase I. Reaction mixtures containing wild-type and/or mutant signal peptidase I were incubated at 37°C for 1 h. The samples were separated by 4 to 20% gradient SDS-PAGE, and the gel was stained by Coomassie brilliant blue. Lane 1, 2 μg of wild-type signal peptidase before incubation; lane 2, 2 μg of wild-type signal peptidase I after incubation; lane 3, 4 μg of mutant S38A after incubation; lane 4, 4 μg of mutant K76A after incubation; lane 5. 2 μg of wild-type signal peptidase I + 4 μg of S38A after incubation; lane 6, 2 μg of wild-type signal peptidase I + 4 μg of K76A after incubation.