Abstract
Chronic kidney disease (CKD) is an independent risk factor for the development of abdominal aortic aneurysm (AAA), as well as for cardiovascular and renal events and all-cause mortality following surgery for AAA or thoracic aortic dissection. In addition, the incidence of acute kidney injury (AKI) after any aortic surgery is particularly high, and this AKI per se is independently associated with future cardiovascular events and mortality. On the other hand, both development of AKI after surgery and the long-term evolution of kidney function differ significantly depending on the type of AAA intervention (open surgery vs. the various subtypes of endovascular repair). Current knowledge regarding AAA in the general population may not be always applicable to CKD patients, as they have a high prevalence of co-morbid conditions and an elevated risk for periprocedural complications. This summary of a Kidney Disease: Improving Global Outcomes Controversies Conference group discussion reviews the epidemiology, pathophysiology, diagnosis, and treatment of Diseases of the Aorta in CKD and identifies knowledge gaps, areas of controversy, and priorities for future research.
Keywords: Aortic diseases, Chronic kidney disease, Acute kidney injury, Abdominal aortic aneurysm, Aortic dissection
1. Introduction
In February 2020, Kidney Disease: Improving Global Outcomes (KDIGO) held the 4th of a series of Controversies Conferences on cardiovascular diseases in patients with chronic kidney disease (CKD) focusing on Central & Peripheral Arterial Diseases in CKD in Dublin, Ireland. The conference covered four large topics: Cerebrovascular Disease, Central Aortic Disease, Renovascular Disease, and Peripheral Arterial Diseases. A summary report1 provided an overview of the conference but was not able to provide in-depth context and full review of these diverse areas.
This report focuses exclusively on Central Aortic Disease, including the associations of CKD with abdominal aortic aneurysm (AAA) and the impact of CKD on post-surgery outcomes; the association of AAA with renal artery stenosis (RAS); the potential impact of CKD on the pathobiology and prognosis of AAA; the approach to the initial diagnostic evaluation of AAA in CKD; the management of AAA in patients with CKD; the incidence, impact on outcomes and prevention strategies of acute kidney injury (AKI) following AAA repair; the post-procedure evaluation of AAA in CKD; the long-term course of kidney function following AAA repair; differences in AAA management in special populations (e.g. acute rupture, elderly, and women); the epidemiology, and management of thoracic aortic dissection in the presence of CKD and the incidence and impact of AKI following thoracic aortic surgery.
2. CKD and abdominal aortic aneurysm: associations and impact on outcomes
2.1 CKD and the risk of incident abdominal aortic aneurysm
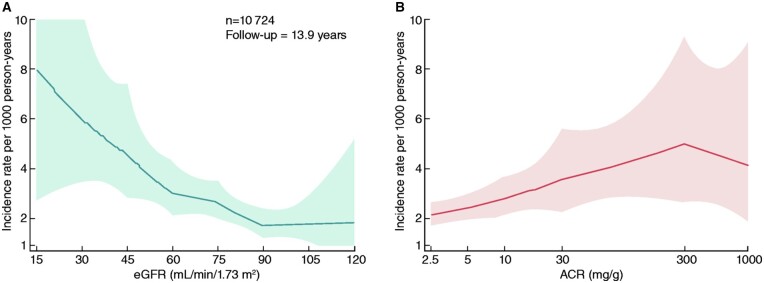
AAA is a progressive disease leading to dilatation of the aortic lumen and is defined as an abdominal aortic diameter of >3.0 cm. The prevalence of AAA in the Western World is estimated at 1–4.5% of men and 0.5% of women at 65–70 years of age.2–6 The prevalence of AAA within CKD patient populations has not been specifically studied. Preliminary data from cross-sectional studies suggest that the prevalence of AAA can be up to 30% higher in individuals with CKD;7,8 however, the cross-sectional nature of such studies did not allow them to determine whether CKD is associated with future risk for AAA development or whether the association is an epidemiologic co-existence driven by shared underlying risk factors. A recent analysis of 10 724 participants in the Atherosclerosis Risk in Communities Study (aged 53–75 years during 1996–1998), a large community-based cohort, evaluated the associations of estimated glomerular filtration rate (eGFR) and urine albumin-to-creatinine ratio (ACR) with incident AAA (diagnosis in outpatient, hospitalization discharge, or death records) over a median follow-up of 13.9 years (Figure 1).9 The demographically adjusted hazard ratios for AAA development were progressively increasing either with descending groups of eGFR (starting from 60–74 mL/min/1.73 m2 compared to the group of ≥90 mL/min/1.73 m2) or with increasing levels of ACR (starting from ACR as low as 10–29 mg/g compared with ACR < 10 mg/g).
Figure 1.
Incidence rate of AAA hospitalizations per 1,000 for CKD measures (A: eGFR, B: ACR), adjusted for age, gender, race, and centre in the Atherosclerosis Risk in Community Study. The demographically adjusted hazard ratio (HR) for AAA development was 4.44 (95% CI 1.58–12.49) for eGFR <30 mL/min/1.73 m2, 3.29 (1.89–5.72) for 30–44 mL/min/1.73 m2, 2.03 (1.29–3.19) for 45–59 mL/min/1.73 m2, and 1.62 (1.11–2.35) for 60–74 mL/min/1.73 m2 compared with eGFR ≥90 mL/min/1.73 m2. Furthermore, the demographically adjusted HR was 2.49 (1.28–4.87) for ACR ≥300 mg/g, 1.99 (1.40–2.83) for 30–299 mg/g, and 1.46 (1.08–1.97) for 10–29 mg/g compared with ACR <10 mg/g. These associations were generally similar after accounting for additional covariates or after stratifying by subgroups. Adapted with permission from Matsushita et al.9
2.2 Associations of pre-surgery kidney function with long-term cardiovascular events and mortality
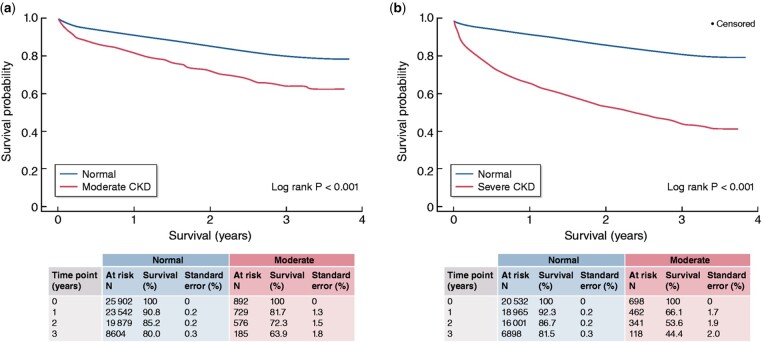
Over the past decade, the effects of pre-surgery kidney function on post-surgery outcomes were also studied. In a prospective cohort study of 383 patients with infrarenal AAA that underwent endovascular aortic aneurysm repair (EVAR), cumulative freedom from the composite end point (death, myocardial infarction, stroke, and peripheral vascular complications), and cumulative survival were progressively lower for declining eGFR groups over 36 months of follow-up. In adjusted Cox-regression analysis, every 1 mL/min/1.73 m2 higher baseline eGFR was associated with a 5% lower likelihood of the composite end-point and a 6% lower likelihood of death.10 Similarly, in a retrospective cohort study of 47 715 patients who underwent AAA repair (of whom 25.7% open repair and 74.3% EVAR), those with moderately (eGFR 30–59 mL/min/1.73 m2) or severely (eGFR <30 mL/min/1.73 m2) impaired kidney function had significantly higher 30-day mortality, a longer length of hospital stay, higher treatment-related costs, and lower 3-year survival compared with individuals without CKD (Figure 2).11
Figure 2.
The effect of pre-existing CKD on survival on 47 715 patients undergoing surgery for AAA (25.7% open repairs and 74.3% EVAR). Blue line: subjects with eGFR > 60 mL/min/1.73 m2 (no CKD, or CKD G1 and G2). Red line, left figure: patients with moderate CKD (G3), right figure: patients with severe CKD (G4 and G5). Adapted with permission from Aranson et al.11
2.3 Associations of pre-surgery kidney function with long-term kidney outcomes
With regard to the association of pre-surgery kidney function with post-surgery renal outcomes, in most relevant studies this was studied in relation to the type of treatment, which is discussed to a greater extent below. In a nested case-matched analysis of AAA patients who underwent open-repair, infrarenal EVAR, or suprarenal EVAR, those with eGFR <60 mL/min/1.73 m2 had greater kidney function loss over 5 years than those without CKD (open repair: −9.8 vs. −6.2 mL/min/1.73 m2, infrarenal EVAR −14.4 vs. −5.1 mL/min/1.73 m2, and suprarenal EVAR −18.8 vs. −15.7 mL/min/1.73 m2, respectively).12 In a cohort of 275 patients with AAA who underwent EVAR, the presence of CKD of G3 or higher was associated with two-fold higher odds for eGFR loss >20% over 9 years of follow-up.13 A three-fold higher risk of patients with pre-existing CKD for eGFR loss >20% or kidney failure was also noted in another cohort of 268 patients with AAA undergoing different types of repair.14
3. Pathophysiology, natural history, and risk of rupture of abdominal aortic aneurysm in patients with and without CKD
AAA is a multifactorial disease. Traditional risk factors for AAA include age, smoking, hypertension, and family history, with a higher prevalence in men across all ages.6 The disease is characterized by infiltration of inflammatory cells including macrophages and lymphocytes into the aortic wall, and associated atherosclerotic processes may also be present. In addition, there is progressive loss of vascular smooth muscle cells (VSMCs) from the aortic wall and degradation of the extracellular matrix due to the production of matrix-degrading enzymes linked to inflammatory cell infiltration and VSMC phenotype change (Figure 3). Resultant structural weakening of the vessel wall renders the aorta more susceptible to rupture.15 However, the exact aetiology of the disease and the factors that precipitate rupture are poorly understood and involve complex interactions between pathological and biomechanical processes.16–18
Figure 3.
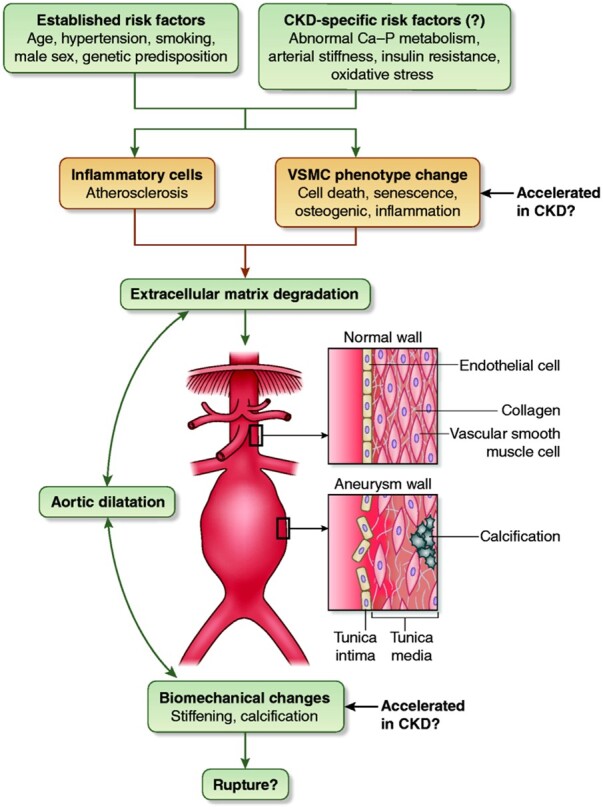
Established and potential CKD-specific risk factors and pathophysiologic mechanisms for AAA development.
CKD and AAA share a number of risk factors including age, hypertension, and smoking. On the other hand, risk factors that are prominent in patients with CKD, such as alterations in calcium-phosphate metabolism, arterial stiffness, oxidative stress, and others may also contribute in AAA development. Hypertension, in particular, plays a central role in the pathogenesis of both diseases. Elevated blood pressure is the most common modifiable risk factor for CKD progression and leads to kidney damage through multiple mechanisms, from glomerular hyperfiltration and proteinuria to hyalinosis of the pre-glomerular vessels causing ischaemia and to direct podocyte injury.19,20 Furthermore, hypertension can promote AAA formation through various pathways, including increased expression of matrix metalloproteinases (MMPs), upregulation of inflammatory responses such as nuclear factor kappaB signalling and others.21,22 Currently, no studies address differences in pathophysiologic mechanisms, natural history, and risk of rupture of AAA between patients with and without CKD or among CKD stages. As discussed above, in the Atherosclerosis Risk in Communities eGFR and albuminuria were independently associated with greater risk of AAA and of greater abdominal aortic diameter.9 Although eGFR and albuminuria may be useful in patient stratification, this study was unable to disentangle mechanisms through which CKD may promote AAA development.23 Therefore, there is a need for research addressing whether features that characterize patients with advanced CKD or on dialysis (e.g. alterations in calcium-phosphate metabolism, arterial stiffness, insulin resistance, oxidative stress, and inflammation) are involved in the development and progression of AAA. It will also be important to compare and contrast the pathological features of the vessel wall in AAA between patients with and without CKD.
The association between vascular stiffening and calcification and the risk of AAA development and rupture is an area of particular interest. These pathologies are common and widespread in ageing and are accelerated across all ages in CKD.24 At the cellular level, calcification is linked to accelerated senescence and death of VSMCs, promoting their conversion to an osteogenic and pro-inflammatory phenotype.25 Premature VSMC ageing is also a feature of AAA.15,26 Studies in non-CKD populations have demonstrated that there is an association between calcification and cardiovascular mortality, all-cause mortality, and rupture in patients with AAA, but more definitive work is required.27–29 Imaging of active calcification using 18F-sodium fluoride was an additive predictor of aneurysm growth and future clinical events, and this or other imaging modalities may be useful in future studies of patients with CKD.18,30 Other studies report associations between arterial stiffness and AAA,31 and it has been postulated that increased MMP activity in both the vasculature and kidney in CKD patients may be particularly involved in AAA development.32 These multi-functional enzymes drive vessel wall remodelling, vascular stiffening, and calcification, as well as kidney fibrosis and were suggested as potential markers to refine risk stratification in CKD;33–35 thus, a possible role of MMPs in the acceleration of aortic abnormalities in these patients should be further investigated.
4. Renal artery stenosis in patients with abdominal aortic aneurysm
The majority of observational studies and clinical trials of AAA or AAA repair do not report rates of renal artery stenosis (RAS). The prevalence of RAS in the angiographic studies of patients with AAA that do report it varies significantly, ranging from 2.6% to 39% (averaging ≈ 30%), depending on the criteria used for case definitions. Relevant parameters include RAS severity (>50–70%; unilateral or bilateral); AAA location (infrarenal vs. suprarenal); presence or absence of CKD; the indication for angiography (angiography for other diseases, angiography for suspected RAS); and the angiographic technique used (arteriography; CT angiography; MR angiography). In older studies with small sample sizes, the prevalence of RAS in patients with AAA was estimated to be around 30%.36 In a recent study with 933 participants, the prevalence of RAS ranged from 5.2% (infrarenal AAA) to 20.3% (suprarenal AAA).37 In another recent study of patients undergoing repair for infrarenal AAA, only 2.6% of patients had RAS, which was defined by stenosis of 70% or more.14 Future studies should assess the prevalence of RAS with angiographic criteria, its functional impact (hypertension control, kidney function), and its the prognostic significance for renal outcomes in patients with AAA.
5. Initial diagnostic evaluation of abdominal aortic aneurysm in patients with CKD
Duplex ultrasonography, computed tomographic angiography (CTA), and magnetic resonance angiography (MRA) are commonly used for the diagnosis of AAA. Duplex ultrasonography refers to B-mode (‘brightness’ mode) grey-scale imaging with pulse-wave Doppler spectral and colour flow analysis. Usually a grey-scale (B-mode ultrasound is sufficient for the initial evaluation and follow-up of an AAA (systolic size measurement of aneurysm extent from outer wall to outer wall in anterior–posterior direction). Additional information can be obtained by colour Doppler ultrasound, which is routinely performed in several countries.38,39 A high-quality examination is dependent on the skill of the technologist and the use of appropriate ultrasound probe with adequate depth of penetration (MHz), fasting patient status, as well as adequate gain and wall filter settings to distinguish true findings from artefact or noise. The main considerations in evaluating AAA include accurate anatomic assessment to identify patients meeting criteria for revascularization (Table 1).40,41 To achieve these goals, B-mode imaging alone is the gold standard, although additional information may be obtained with pulse-wave Doppler spectral and colour flow analysis (e.g. presence and extent of mural thrombus).38,39,42
Table 1.
Comparison of pros and cons related to various imaging modalities available for the detection and initial evaluation of AAA
| Pros | Cons | |
|---|---|---|
| Duplex ultrasound | Operator dependent, may be limited by habitus (obesity), bowel gas (fasting):
|
|
| CT angiography | Gold standard for pre-op planning:
|
|
| MR angiography | Pre-op planning possible:
|
|
| Catheter-based angiography |
|
|
AAA, abdominal aortic aneurysm; CO2, carbon dioxide; GDCA, gadolinium-based contrast agent; IVUS, intravascular ultrasound; NSF, nephrogenic systemic fibrosis; Sn/Sp, sensitivity/specificity.
It is important to note, however, that ultrasound alone is not sufficient for procedural planning, and additional imaging, preferably with CTA, is almost always necessary given the complexity of these procedures.40,41 Recent data and consensus statements indicate that the risk of contrast-induced nephropathy (CIN) may have been overstated historically and should not deter from proper diagnosis and treatment of AAA in patients with CKD.43 The amount of contrast used with modern CTA is considerably less than in prior years. It is also important to note that CIN risk prediction tools are available to help predict which patients undergoing EVAR procedures may experience adverse events from iodinated contrast.44 The Mehran risk-prediction model, which is most widely adopted for CIN in coronary intervention patients, seems to have the best discriminative ability among EVAR patients.45 Magnetic resonance angiography (MRA) is less useful in the initial or pre-procedural evaluation of AAA due to motion artefact, resolution, and cost, although gadolinium-based contrast agent (GBCA) imaging may improve the quality of the examination and is no longer a major risk for patients with CKD, as long as group II agents are used.40,41,46 According to the American College of Radiology, macrocyclic ionic GBCAs classified in group II (gadobenate dimeglumine, gadobutrol, gadoterate meglumine, or gadoteridol) have higher stability from the dissociation of gadolinium than linear and non-ionic agents.47,48 A meta-analysis on the risk of nephrogenic systemic fibrosis (NSF) concluded that the risk of NSF in CKD G4 or G5 receiving a Group II GBCA is less than 0.07%.49 Thus, the potential diagnostic harms of withholding group II GBCAs for indicated MRI examinations may outweigh the risk of NSF.
6. Management of abdominal aortic aneurysm in patients with CKD
6.1 Indications for treatment and available modalities for AAA repair
There is no proven medical therapy that reduces the risk of AAA rupture. Open surgical and endovascular repair are the only treatments shown to decrease AAA-specific mortality.50 However, both have a number of complications, including acute and chronic kidney dysfunction. The indications for AAA treatment in the elective and emergency settings do not differ for patients who have established CKD.50 When an AAA measures less than 55 mm in maximal antero-posterior diameter, the rupture risk is less than the risk of surgery.51 Men with an AAA diameter >55 mm are considered for elective AAA surgery; in women, the threshold for considering elective AAA repair may be around 50 mm. Patients with symptoms secondary to the AAA (e.g. pain) and those who present with a rupture are considered for emergency repair.50 Those with an increase in diameter >10 mm in 1 year should be referred to a surgeon. The presence of CKD should be taken into account during pre-operative risk assessment, since CKD is associated with a higher risk of post-surgery AKI, long-term eGFR decline, cardiovascular events, and mortality, as discussed extensively above.
Endovascular aneurysm repair (EVAR) has superior short-term outcomes compared with open surgery and has become the treatment of choice for many patients.50 However, there are no specific data to support offering EVAR over open surgery in individuals with CKD; anatomical and other patient-related parameters should be taken into account when making that decision. Typical infrarenal AAA have a proximal aortic neck that provides an adequate landing zone for the endovascular device; juxtarenal aneurysms do not have this zone, and the aneurysm involves the infrarenal abdominal aorta adjacent to or including the lower margin of renal artery origins; suprarenal and thoraco-abdominal aneurysms extend above and beyond the orifice of renal arteries (Figure 4).52
Figure 4.
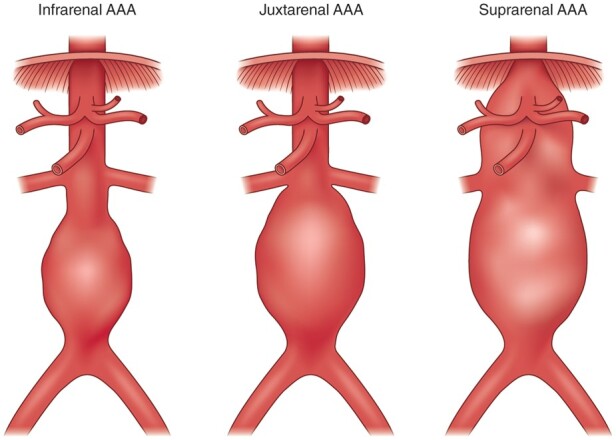
Classification of abdominal aortic aneurysms. Infrarenal aneurysms have a proximal aortic neck that provides an adequate landing zone for the endovascular device; in juxtarenal aneurysms the aneurysm involves the infrarenal abdominal aorta adjacent to or including the lower margin of renal artery origins; suprarenal AAAs and thoraco-abdominal aneurysms extend above the orifice of renal arteries.
Based on the exact anatomy of the aneurysm, there are several potential modes of open or endovascular AAA reconstruction. Open AAA repair can be performed with a suprarenal or an infrarenal aortic clamp based on the anatomy of the proximal AAA neck. Suprarenal clamping is associated with higher morbidity and AKI rates,53,54 which are expected, given the ischaemic insult to the kidneys. Endovascular repair for a typical infrarenal AAA (i.e. the proximal aortic neck of the AAA provides an adequate landing zone) can be performed using an infrarenal device (i.e. standard EVAR), which may or may not have suprarenal fixation modalities (e.g. bare stents or hooks).53 Suprarenal fixation is meant to decrease the chance of device migration and potential endoleak over the long term. Aneurysms with a ‘hostile’ proximal neck (where a standard infrarenal EVAR device would not provide adequate seal), juxtrarenal, suprarenal, or thoraco-abdominal aneurysms cannot be treated with standard EVAR ‘off the shelf’ devices. More complex forms of EVAR have been devised to address these anatomies, such as fenestrated EVAR (fEVAR) or branched EVAR (bEVAR).53 Use of these complex endovascular procedures usually requires more contrast than standard infrarenal EVAR and involves a high risk for renal artery occlusion (estimated at 2.3% for fEVAR and 9.6% for bEVAR) or stenosis, given that covered stents are deployed in the actual renal vasculature.55
7. Acute kidney injury after abdominal aortic aneurysm surgery: incidence, risk factors, impact on outcomes, and prevention strategies
7.1 Incidence of AKI following interventions for AAA
AKI after elective AAA surgery is an important complication.56 In original studies exploring this association AKI incidence ranged widely, due to the variation of criteria used (change in serum creatinine levels, decrease in creatinine clearance or eGFR, and others).57 In recent years, the use of contemporary criteria for AKI definition, such as the Risk-Injury-Failure-Loss-End-stage (RIFLE), Acute-Kidney-Injury-Network (AKIN), and KDIGO criteria has enabled better comparison among studies.58 The majority of observational studies using contemporary criteria examined AKI incidence after elective EVAR for infrarenal AAA, and reported incidence around 15–20%, most of which was Stage 1 AKI (Table 2).53,59–68 In the only study using the AKIN and KDIGO criteria and also including urine output measurements, Saratzis et al. reported a postoperative AKI incidence of 18.8% in 149 patients undergoing EVAR.62 In 947 patients undergoing elective EVARs for infrarenal AAA, AKI incidence was 18% using the AKIN and KDIGO criteria.63 Studies examining AKI incidence between different modes of AAA treatment are discussed in section 7.4.
Table 2.
Studies reporting incidence of acute kidney injury (AKI) after (a) elective infrarenal endovascular aneurysm repair (EVAR) and (b) elective fenestrated endovascular aneurysm repair (fEVAR) and branched repairs using standardized AKI reporting criteria
| References (b) |
Type | Date | n EVAR | AKI criterion | AKI incidence (%) | n AKI | n AKI stage > 2 | Dialysis | Urine output available |
|---|---|---|---|---|---|---|---|---|---|
| (a) | |||||||||
| Pirgakis et al.59 | Retrospective | 2014 | 87 | AKIN | 17 | 15 | None | 1 | No |
| Ueta et al.60 | Prospective | 2014 | 47 | AKIN | 13 | 6 | Stage 2: 1 | None | No |
| Pisimisis et al.61 | Retrospective | 2013 | 208 | RIFLE | 17 | 36 | Not available | Not available | No |
| Saratzis et al.62 | Prospective | 2015 | 149 | AKIN and KDIGO | 19 | 28 | Stage 2: 3 | None | Yes |
| Saratzis et al.63 | Retrospective | 2015 | 947 | KDIGO | 18 | 167 | Stage 2: 12; Stage 3: 2 | None | No |
| Saratzis et al.64 | Retrospective | 2016 | 484 | AKIN | 12 | 58 | Not available | None | No |
| Obata et al.65 | Prospective | 2016 | 95 | AKIN | 9.5 | 9 | Stage 2: 1 | None | No |
| Lee et al.66 | Retrospective | 2017 | 78 | KDIGO | 14 | 11 | None | None | No |
| Saratzis et al.67 | Prospective (pilot randomized trial) | 2018 | 58 | KDIGO | 21 | 12 | None | None | Yes |
| Zabrocki et al.68 | Retrospective | 2018 | 91 | KDIGO | 13 | 12 | None | None | No |
| Saratzis et al.53 | Prospective multicentre | 2019 | 139 | KDIGO | 18 | 13 | None | None | Yes |
| Saratzis et al.69 | Retrospective | 2015 | 58 | KDIGO | 28 | 16 | None | None | No |
| Sailer et al.70 | Retrospective | 2016 | 158a (fEVAR and branched repairs) | AKIN | 27a | 43 | Not available | Not available | No |
| Ducasse et al.71 | Retrospective | 2016 | 25 | KDIGO | 32 | 8 | 2 | 2 | No |
| Tran et al.72 | Retrospective | 2016 | 110 | RIFLE | 23 | 25 | 10 | 2 | No |
| Tinelli et al.73 | Retrospective | 2017 | 102a (fEVAR and branched repairs) | RIFLE | 20a | 20 | 5 | 5 | No |
| Wang et al.74 | Retrospective | 2019 | 120 | RIFLE | 20 | 24 | 4 | 4 | No |
| Saratzis et al.53 | Prospective multicentre | 2019 | 30 | KDIGO | 27 | 8 | None | None | Yes |
| Khoury et al.75 | Prospective | 2020 | 186a (fEVAR and branched repairs) | RIFLE | 15a | 27 | Not available | Not available | Not available |
Results for fEVAR are reported together with branched repairs.
7.2 Risk factors for AKI following interventions for AAA
Epidemiologic studies examining risk factors for AKI following AAA interventions are scarce. The lack of uniform reporting of AKI, the complex pathophysiology of factors involved, and the absence of information on the perioperative volume status of patients largely prohibit valid analyses.57 In the aforementioned cohort of 947 patients undergoing elective EVARs, pre-operative eGFR, and CKD >G2 were the only independent predictors of AKI among a wide set of factors studied, including age, sex, major co-morbidities, aneurysm diameter, the volume of contrast medium used, and others.63 In a recent prospective study of 300 patients undergoing different types of AAA repair, older age, baseline eGFR, and ischaemic heart disease were the main predictors of AKI after infrarenal EVAR and open repair.53
7.3 AKI and impact on long-term kidney function, cardiovascular outcomes, and mortality
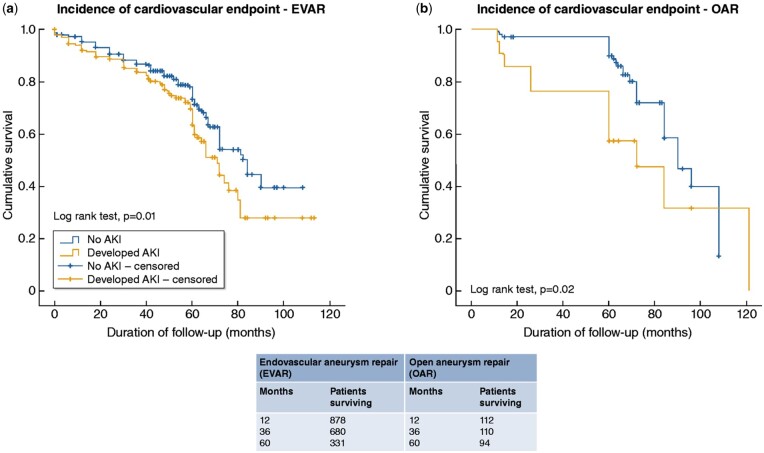
Existing data suggest that AKI development following AAA surgery is an independent risk factor for eGFR decline, as well as for cardiovascular events and mortality.14,62,63 In a recent study of 266 individuals undergoing AAA repair with either EVAR or open surgery, AKI was independently associated with eGFR decline >20% and/or kidney failure during follow-up for both types of repair.14 In the aforementioned study of 149 elective EVARs from Saratzis et al.62 patients who developed AKI were more likely to die or develop cardiovascular complications over 33 months of follow-up in univariate analyses, and AKI was independently associated with death and cardiovascular morbidity in exploratory adjusted survival analyses. In another cohort of 1068 individuals, of which 947 underwent EVAR and 121 open repair for AAA, AKI following intervention was independently associated with a 1.7-fold higher risk of cardiovascular events during a median follow-up of 62 months (Figure 5).63 Evidence from other clinical areas suggests that AKI contributes to long-term kidney function loss through multiple structural changes, including glomerulosclerosis and tubulointerstitial fibrosis.77 However, it is not known whether AKI is pathophysiologically involved in the acceleration of cardiovascular disease or is simply a marker of occult cardiovascular burden in these individuals.57
Figure 5.
Kaplan–Meier curves indicating cumulative freedom from the combined cardiovascular end-point for patients with or without AKI after EVAR (left figure) or open repair (right figure) for AAA. From Saratzis A et al.,63 with permission.
7.4 Effects of AAA treatment modality on AKI incidence
As discussed above, AKI develops in 15–20% of patients having elective EVAR for infrarenal AAA.53,59–68,76 Whether open repair is associated with a lower, equal, or higher AKI risk is not yet clear. Saratzis et al.63 previously reported an incidence of AKI of 18% and 17% of patients undergoing EVAR and open repair, respectively, for infrarenal AAA. In a study from Al-Adas et al., patients undergoing open repair had a 1.6-fold higher risk for immediate postoperative AKI compared with those undergoing EVAR,78 whereas a UK cohort53 had AKI rates at 18% for infrarenal EVAR, 39% for infrarenal AAA open repair, and 37% for juxtarenal AAA open repair.53 In two studies using contemporary criteria to define AKI, patients undergoing EVAR with suprarenal fixation had similar AKI rates as those undergoing infrarenal fixation.79 Finally, small studies suggest that the risk of AKI with fEVAR or bEVAR procedures is typically higher than with standard EVAR, i.e., usually at around 25–30% of patients (Table 2).53,69–75
7.5 Peri-procedural management for AKI prevention in patients with AAA
There is currently no evidence-based strategy in the context of open AAA surgery or EVAR that has been proven to reduce the risk of AKI or subsequent longer-term renal decline.57,80 No RCT examining prevention of renal complications post-AAA surgery has focused on CKD patients.57 Current guidance for patients at high risk for AKI based on RCTs from relevant clinical areas suggests perioperative intravenous fluid administration using crystalloid solutions for those with an eGFR <40 mL/min/1.73 m2 or those with a history of kidney transplantation or a solitary kidney.81 So far, various interventions for AKI reduction have been tested, including N-acetylcysteine;82 ischaemic pre-conditioning;83 high-dose intrarenal artery infusions of fenoldopam delivered via a left brachial access;84 intravenous fluids with bicarbonate;85 administration of anti-oxidants (e.g. vitamin C).86 The majority of these interventions were assessed in under-powered exploratory studies, often using inconsistent AKI reporting criteria. A consensus group on AKI following EVAR in the UK reported a pilot RCT investigating a large bolus dose of bicarbonate to alkalinize patients’ urine prior to commencing EVAR together with a standardized regimen of aggressive intravenous volume expansion with crystalloid solutions.67 Participants who received this two-step intervention (even those with heart failure or advanced CKD) did not experience adverse events, and the strategy was easy to implement; a larger RCT to test this intervention is currently under development.
8. Post-procedure evaluation of abdominal aortic aneurysm in patients with CKD
Following revascularization, the major considerations for follow-up include repeat contrast imaging to monitor for complications in patients with EVAR as compared to open repair. As has been stated previously, the benefit of improved in-hospital mortality after EVAR is offset by higher long-term complications including endoleak (Figure 6), device migration, and continued aneurysm expansion requiring repeat intervention. Traditionally, the surveillance protocol following EVAR included CTA at 1-month, 6-months, 1 year, and then annually paired with duplex ultrasound imaging. More recent data and US guidelines suggest that the 6-month imaging exams may be dropped if the 1-month evaluation is without complications.41,87 Alternatively, the European guidelines advocate for the use of duplex ultrasound with non-contrast CT and abdominal radiographs at any time after EVAR in patients with CKD.40 CTA is reserved for suspected endoleak in this algorithm. In some centres, contrast-enhanced duplex ultrasonography (CEUS) is available which shows promise of excellent sensitivity and specificity for the detection of endoleaks88 and may replace CTA for endoleak detection in the future.
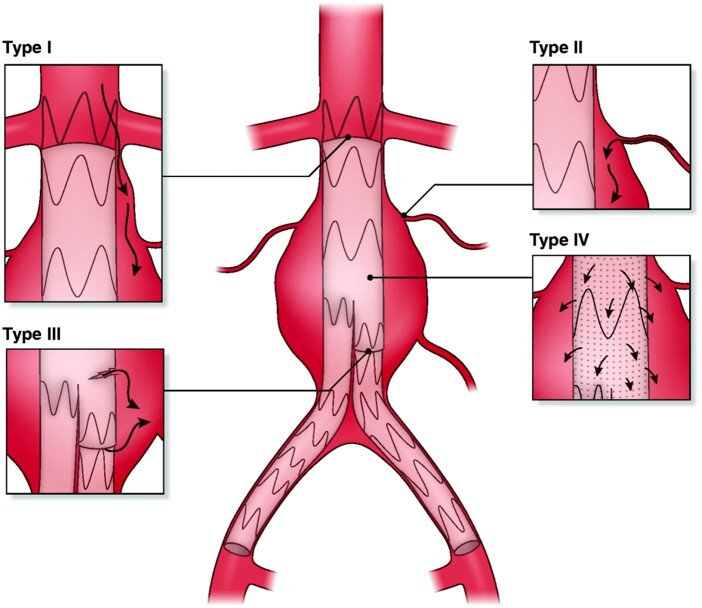
Figure 6.
The types of endoleaks after endovascular aortic repair: Type I, leak at the proximal or distal landing of the graft; Type II, leak via branches (e.g. lumbar artery) into the aneurysm sac; Type III, modular defect or tearing of the graft material; Type IV, graft porosity.
9. Long-term kidney function after abdominal aortic aneurysm surgery
With regard to the effects of the type of repair to mid- and long-term kidney function, early observational studies suggested that patients with infrarenal AAA that underwent EVAR with suprarenal fixation (i.e. using an infrarenal ‘off-the-shelf’ EVAR device with suprarenal fixation modalities) experienced a greater eGFR decline over the 1st and 2nd-year post-surgery than those having EVAR with no suprarenal fixation.89,90 A subsequent nested case-matched analysis of 726 patients compared 121 patients undergoing open-repair case-matched for age, sex, smoking, diabetes, baseline eGFR with patients undergoing suprarenal and infrarenal fixation EVAR (242 in each group) and 121 patients without AAA undergoing carotid endarterectomy (CEA) as a control group. Over 5 years of follow-up, eGFR declined at least twice as much among patients who underwent EVAR with suprarenal fixation compared with all other groups: 7.4, 8.2, 16.9, and 5.4 mL/min/1.73 m2 for open repair, infrarenal EVAR, suprarenal EVAR, and carotid endarterectomy, respectively (Figure 7).12 Another retrospective study including 317 patients with open repair and 358 with EVAR showed that eGFR decline in the long-term was almost two-times greater in patients undergoing EVAR.78 A recent meta-analysis reporting on eGFR changes at 1 and 5 years suggested that EVAR with suprarenal fixation does not lead to a significantly greater drop in kidney function compared with infrarenal fixation at one year; however, there is a greater loss of eGFR over 5 years.79
Figure 7.
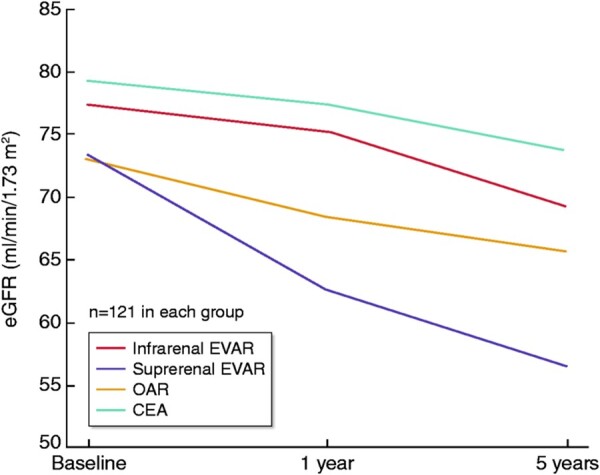
Long-term kidney function after AAA repair according to treatment type. OAR, patients with open repair; EVAR (infra), patients infrarenal EVAR; EVAR (supra), patients with suprarenal EVAR; CEA patients with carotid endarterectomy, without AAA serving as the control group. From Saratzis et al.,12 with permission.
10. Thoracic aortic dissection and kidney disease
10.1 CKD and thoracic aortic dissection: associations and impact on outcomes
Thoracic aortic dissection is a rare but serious cardiovascular disease. According to the Stanford classification, which is commonly used, dissections involving the ascending aorta are classified as Type A and those without ascending aorta involvement as Type B.91 There are few data on the epidemiology, natural course, and complications of aortic dissection in patients with CKD. In previous reports, the prevalence of CKD was noted at 8.5–10% of patients with acute aortic dissection.92,93 Recently, Reutersberg et al. reported registry data collected in Germany between 2006 and 2014 from patients presenting with Type A (n = 14 911) and Type B (n = 5622) acute aortic dissection, for surgery and/or intervention. In this study, CKD was present in 19.3% of Type A and 20.4% of Type B patients.94 Currently, no longitudinal study has particularly evaluated whether CKD is a risk factor for aortic dissection development.
In the German Registry study, pre-existing CKD was not associated with mortality in patients with Type A dissection. In patients with Type B dissection, however, the prevalence of CKD was higher in non-survivors (23.9% vs. 20% in survivors, P = 0.039).94 Pre-existing CKD was also an independent predictor of mortality in the study by Hoogmoed et al.,92 but not in a report from the International Registry of Acute Aortic Dissections (IRAD) that included 1034 patients.93
A recent retrospective study of all patients with renal failure on dialysis in the USA who underwent open proximal aortic repair with the diagnosis of non-ruptured thoracic aortic aneurysm (n = 325) or type A aortic dissection (n = 461) during the years 1987–2015, showed perioperative mortality (in-hospital or 30-day mortality) of 12.6% and 24.3%, and 10-year mortality of 81% and 87.9%, respectively.95 In patients with type A aortic dissection, age ≥65 years, heart failure, and diabetes were independently associated with worse 10-year mortality. This study affirmed the feasibility of emergency surgery for acute type A dissections but also highlighted the need for careful patient selection in the elective repair of proximal thoracic aneurysm for dialysis-dependent patients.
10.2 Management of thoracic aortic dissection in patients with CKD
Currently, there are no data supporting differences in treatment practices of aortic dissection based on the presence of CKD. Although Type A dissections are almost always treated surgically, Type B dissections are mostly treated interventionally, especially if visceral or renal arteries are compromised.96 This was the case also in the aforementioned German registry, where the majority of Type A patients underwent open surgery, whereas most of Type B had endovascular procedures.94 An early intervention is the preferred treatment to minimize ischaemic time of visceral organs and the kidneys, and often it is the only option to provide a reasonable chance of survival in patients with acute aortic dissection. However, in the case of advanced age or extensive pre-existing comorbidities, the risk of complications may justify non-intervention, because in these patients prognosis is dismal. Medical treatment with aggressive blood pressure lowering for Type B dissections is recommended in uncomplicated cases or patients with a prohibitive risk profile.96
10.3 Acute kidney injury after thoracic aortic dissection: incidence, risk factors, and impact on outcomes
In the German Registry study, the need for postoperative kidney replacement therapy was extremely high (24.2%) in Type A and high (8.2%) in Type B patients94 due to the multiplicity of risk factors, emergent setting, and complexity of the operations. In another retrospective study, Hoogmoed et al. reviewed 478 patients with Acute Type B aortic dissection; patients on dialysis were excluded. 52.7% of patients experienced AKI (27.2% Stage I, 14.9% Stage II, and 10.7% Stage III). Independent predictors for AKI were CKD, renal malperfusion, congestive heart failure, hypertension, visceral malperfusion, and limb malperfusion. AKI was associated with a longer hospital stay, and, in Stages II and III only, with reduced late survival but not with late aortic events during follow-up.92 Other studies also suggest that renal dysfunction on admission and renal artery involvement contribute to AKI development, while AKI per se is associated with a higher risk of in-hospital complications.97 AKI was also identified as an independent predictor for mortality in the IRAD study.93
Finally, a recent observational study in 129 individuals that received endovascular repair for acute type B aortic dissection reported that 16.3% of the patients had RAS; these individuals had a higher incidence of AKI and lower eGFR both pre-operatively and 1-month post-operatively than individuals without RAS (81.7 ± 23.8 vs. 96.0 ± 20.0 mL/min, P = 0.017).98
11. Differences in management in special conditions and populations
Management of patients with aortic pathology and CKD can be broken down into those with elective conditions and those with acute aortic syndromes (dissections, intramural haematomas, penetrating aortic ulcers, symptomatic, or ruptured aortic aneurysms). For the latter patients, the presence of CKD is an important factor in counselling the patient and family as to peri-procedural risk and mortality, as well as potential worsening of kidney function towards kidney failure. As discussed above, however, there is currently no evidence restricting the use of open or endovascular treatments for the above conditions in patients with CKD. In the absence of specific evidence, one can extrapolate from the literature that the overall short-term perioperative morbidity is generally lower with endovascular repair use in emergency situations.50 This is most clear for ruptured infrarenal AAA abdominal aortic aneurysms, where there is a clear shift towards the use of EVAR techniques worldwide.99
The complexity of the procedure is also important for treatment decisions; a patient with severe CKD and a penetrating aortic ulcer with contained rupture who could be treated with EVAR is very different from one needing an urgent open thoraco-abdominal aneurysm repair. Furthermore, in patients with advanced age (i.e. >75–80 years), decisions on treatment and treatment modality of both thoracic and aortic diseases should be made on an individualized approach based on a risk-benefit basis, as the very elderly are often excluded from relevant studies, and surgery may not increase overall life expectancy.100,101 For younger and relatively fit patients with AAA, open surgical repair may be preferred, as it is associated with slightly better long-term survival101 and better long-term preservation of kidney function in relation to suprarenal EVAR.12
Finally, women tend to be older, have smaller aneurysms, and higher prevalence of CKD compared to men.102,103 When undergoing AAA repair, women are less likely to undergo EVAR and have higher rates of procedural complications and, in some studies, in-hospital mortality.102–104 These factors need to be taken into account when deciding about treatment.
12. Limitations of existing evidence and issues for further research
In recent years, the literature on the associations of aortic diseases with CKD or AKI has been growing. However, the majority of data come from observational studies, several limitations prohibit drawing definite conclusions, and several aspects are open to future research. With regard to long-term evolution of kidney function or incidence of AKI after AAA or thoracic dissection surgery, outcomes may be affected by factors not adequately assessed by existing studies, such as anatomic complexity, clamp time, clamp location in AAA (suprarenal vs. infrarenal), intentional occlusion of accessory renal arteries, and others. Studies on long-term kidney function after EVAR may reflect treatment practices in the early era of the procedure (15–20 years ago) with regard to choice of fixation, procedural time, increased contrast volume, and increased complication rates; such practices may have substantially changed over time. Furthermore, techniques and experience for AAA repair may vary substantially in different parts of the world.
With regard to the exact treatment types, the accurate pathophysiology of eGFR loss following EVAR (especially with suprarenal fixation) remains to be fully established: bare metal material covering the orifice of renal arteries, wire placement during the procedure, inflammation of the aortic sack, and micro-emboli may be important. In addition, most of the relevant evidence refers to outcomes after correction of infrarenal AAA and not of AAA involving the orifice of renal arteries or thoraco-abdominal aneurysms with complicated anatomy, such as those with false channel affecting renal arteries. The use of fenestrated or physician-modified grafts is increasing in several countries for simple or complex aneurysm types. The associations of EVAR with such grafts with long-term kidney function are poorly studied.
Other gaps in knowledge requiring further investigation include the pathophysiologic mechanisms through which CKD may affect AAA and thoracic aortic dissection development (including the role of the severity of atherosclerotic lesions above the AAA), the prevalence of RAS and its impact on outcomes in patients with AAA, the mechanisms through which AKI post-surgery for AAA or thoracic dissection affects cardiovascular events and all-cause mortality, and the long-term evolution of kidney function following surgery for aortic dissection. Randomized trials are also needed to assess several questions, such as the effects of different post-surgery diagnostic protocols on long-term kidney function, the effects of different treatment modalities for AAA on long-term kidney and cardiovascular outcomes, and the effects of AKI prevention strategies in CKD patients.
13. Conclusions
Chronic kidney disease and aortic diseases are tightly linked with bi-directional associations. The presence of CKD is an independent risk factor for the development of AAA and is associated with adverse cardiovascular and kidney outcomes and all-cause mortality following surgery for AAA or thoracic aortic disease. In parallel, the incidence of AKI after any type of aortic intervention is particularly high, and this AKI is independently associated with future cardiovascular events and mortality. On the other hand, the type of AAA surgery (open vs. the various subtypes of endovascular repair) directly affects the rates of post-procedural AKI and the long-term course of kidney function. In contrast to emerging epidemiological data, available evidence on the pathophysiology, proper diagnosis, and treatment of AAA or thoracic aortic dissection specifically in the context of CKD is limited. This is also the case for the association of AAA with RAS. As the prevalence rates of CKD and aortic diseases are continuously increasing, observational studies and clinical trials on all the above fields are urgently needed to delineate the complex associations between these entities for the benefit of our patients.
Authors’ contributions
C.H., M.C., H.R., K.J.: conception of the work; P.S., S.M., A.S., D.K.-D., P.M., C.S., A.H., D.E., U.T.: manuscript drafting; C.H., M.C., M.J., W.W., H.R., and K.J.: critical editing for intellectual content.
Funding
The conference was sponsored by Kidney Disease Improving Global Outcomes and supported in part by unrestricted educationalgrants from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Janssen, Lilly, and Vifor Fresenius Medical Care Renal Pharma.
Contributor Information
Pantelis Sarafidis, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Konstantinoupoleos 49, Thessaloniki GR54642, Greece.
Sven Martens, Department of Cardiothoracic Surgery—Division of Cardiac Surgery, Münster, University Hospital, Universitätsklinikum, Münster, Germany.
Athanasios Saratzis, Department of Vascular Surgery, Leicester University Hospital and NIHR Leicester Biomedical Research Centre, Leicester, UK.
Daniella Kadian-Dodov, Zena and Michael A. Wiener Cardiovascular Institute, and Marie-Josée and Henry R. Kravis Center for Cardiovascular Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Patrick T Murray, Department of Nephrology, School of Medicine, University College Dublin, Dublin, Ireland.
Catherine M Shanahan, School of Cardiovascular Medicine and Sciences, King's College London, London, UK.
Allen D Hamdan, Division of Vascular and Endovascular Surgery, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Daniel T Engelman, Heart, Vascular & Critical Care Services Baystate Medical Center, and University of Massachusetts Medical School-Baystate, Springfield, MA, USA.
Ulf Teichgräber, Department of Radiology, Jena University Hospital, Friedrich-Schiller-University, Jena, Germany.
Charles A Herzog, Division of Cardiology, Department of Medicine, Hennepin County Medical Center and University of Minnesota, Minneapolis, MN, USA; Chronic Disease Research Group, Minneapolis Medical Research Foundation, Minneapolis, MN, USA.
Michael Cheung, KDIGO, Brussels, Belgium.
Michel Jadoul, Cliniques Universitaires Saint Luc, Université Catholique de Louvain, Brussels, Belgium.
Wolfgang C Winkelmayer, Selzman Institute for Kidney Health, Section of Nephrology, Department of Medicine, Baylor College of Medicine, Houston, TX, USA.
Holger Reinecke, Department of Cardiology I: Coronary and peripheral vessel disease, heart failure; Münster University Hospital, Universitätsklinikum, Münster, Germany.
Kirsten Johansen, Division of Nephrology, Hennepin County Medical Center and University of Minnesota, Minneapolis, MN, USA.
Data Availability
This is a conference report and no original data are available.
References
- 1. Johansen K, Garimella P, Hicks C, Kalra PA, Kelly D, Martens S, Matsushita K, Sarafidis P, Sood M, Herzog CA, Cheung M, Jadoul M, Winkelmayer WC, Reinecke H, Conference Participants . Central and peripheral arterial diseases in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2021;100:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cosford PA, Leng GC.. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev 2007;2:CD002945. [DOI] [PubMed] [Google Scholar]
- 3. Svensjo S, Bjorck M, Gurtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A.. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation 2011;124:1118–1123. [DOI] [PubMed] [Google Scholar]
- 4. Barba Á, Vega de Céniga M, Estallo L, de la Fuente N, Viviens B, Izagirre M.. Prevalence of abdominal aortic aneurysm is still high in certain areas of southern Europe. Ann Vasc Surg 2013;27:1068–1073. [DOI] [PubMed] [Google Scholar]
- 5. Svensjö S, Björck M, Wanhainen A.. Current prevalence of abdominal aortic aneurysm in 70-year-old women. Br J Surg 2013;100:367–372. [DOI] [PubMed] [Google Scholar]
- 6. Lindholt JS, Diederichsen AC, Rasmussen LM, Frost L, Steffensen FH, Lambrechtsen J, Urbonaviciene G, Busk M, Egstrup K, Kristensen KL, Behr Andersen C, Søgaard R.. Survival, prevalence, progression and repair of abdominal aortic aneurysms: results from three randomised controlled screening trials over three decades. Clin Epidemiol 2020;12:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chun KC, Teng KY, Chavez LA, Van Spyk EN, Samadzadeh KM, Carson JG, Lee ES.. Risk factors associated with the diagnosis of abdominal aortic aneurysm in patients screened at a regional Veterans Affairs health care system. Ann Vasc Surg 2014;28:87–92. [DOI] [PubMed] [Google Scholar]
- 8. Alcorn HG, Wolfson SK, Sutton-Tyrrell K, Kuller LH, O'Leary D.. Risk factors for abdominal aortic aneurysms in older adults enrolled in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 1996;16:963–970. [DOI] [PubMed] [Google Scholar]
- 9. Matsushita K, Kwak L, Ballew SH, Grams ME, Selvin E, Folsom AR, Coresh J, Tang W.. Chronic kidney disease measures and the risk of abdominal aortic aneurysm. Atherosclerosis 2018;279:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saratzis A, Sarafidis P, Melas N, Saratzis N, Kitas G.. Impaired renal function is associated with mortality and morbidity after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2013;58:879–885. [DOI] [PubMed] [Google Scholar]
- 11. Aranson NJ, Lancaster RT, Ergul EA, Conrad MF, LaMuraglia GM, Kwolek CJ, Cambria RP, Patel VI.. Chronic kidney disease class predicts mortality after abdominal aortic aneurysm repair in propensity-matched cohorts from the medicare population. Ann Surg 2016;264:386–391. [DOI] [PubMed] [Google Scholar]
- 12. Saratzis A, Bath MF, Harrison S, Sayers RD, Mahmood A, Sarafidis P, Bown MJ.. Long-term renal function after endovascular aneurysm repair. Clin J Am Soc Nephrol 2015;10:1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Charles ER, Lui D, Delf J, Sayers RD, Bown MJ, Sidloff D, Saratzis A.. Editor’s choice - the impact of endovascular aneurysm repair on long term renal function based on hard renal outcomes. Eur J Vasc Endovasc Surg 2019;58:328–333. [DOI] [PubMed] [Google Scholar]
- 14. Zabrocki L, Marquardt F, Albrecht K, Kribben A, Herget-Rosenthal S.. Permanent decline of renal function after infrarenal abdominal aortic aneurysm repair-frequency and risk factors. Ann Vasc Surg 2018;47:272–278. [DOI] [PubMed] [Google Scholar]
- 15. Riches K, Clark E, Helliwell RJ, Angelini TG, Hemmings KE, Bailey MA, Bridge KI, Scott DJA, Porter KE.. Progressive development of aberrant smooth muscle cell phenotype in abdominal aortic aneurysm disease. J Vasc Res 2018;55:35–46. [DOI] [PubMed] [Google Scholar]
- 16. Leemans EL, Willems TP, van der Laan MJ, Slump CH, Zeebregts CJ.. Biomechanical indices for rupture risk estimation in abdominal aortic aneurysms. J Endovasc Ther 2017;24:254–261. [DOI] [PubMed] [Google Scholar]
- 17. Raaz U, Zöllner AM, Schellinger IN, Toh R, Nakagami F, Brandt M, Emrich FC, Kayama Y, Eken S, Adam M, Maegdefessel L, Hertel T, Deng A, Jagger A, Buerke M, Dalman RL, Spin JM, Kuhl E, Tsao PS.. Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation 2015;131:1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kolipaka A, Illapani VSP, Kenyhercz W, Dowell JD, Go MR, Starr JE, Vaccaro PS, White RD.. Quantification of abdominal aortic aneurysm stiffness using magnetic resonance elastography and its comparison to aneurysm diameter. J Vasc Surg 2016;64:966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sarafidis PA, Sharpe CC, Wood E, Blacklock R, Rumjon A, Al-Yassin A, Ariyanayagam R, Simmonds S, Fletcher-Rogers J, Vinen K.. Prevalence, patterns of treatment, and control of hypertension in predialysis patients with chronic kidney disease. Nephron Clin Pract 2012;120:147– 155. [DOI] [PubMed] [Google Scholar]
- 20. Seccia TM, Caroccia B, Calò LA.. Hypertensive nephropathy. Moving from classic to emerging pathogenetic mechanisms. J Hypertens 2017;35:205–212. [DOI] [PubMed] [Google Scholar]
- 21. Zhang W-H, Qiao C-H, Zhang X, Luo H, Sun X-K.. The expression of MMP-7 in serum and aneurysm tissues of patients with abdominal aortic aneurysm associated with hypertension and the clinical efficacy of endovascular exclusion. Eur Rev Med Pharmacol Sci 2017;21:4623–4631. [PubMed] [Google Scholar]
- 22. Shiraya S, Miwa K, Aoki M, Miyake T, Oishi M, Kataoka K, Ohgi S, Ogihara T, Kaneda Y, Morishita R.. Hypertension accelerated experimental abdominal aortic aneurysm through upregulation of nuclear factor kappaB and Ets. Hypertension 2006;48:628–636. [DOI] [PubMed] [Google Scholar]
- 23. Bellasi A, Papagni S, Di Lullo L.. Chronic kidney disease: a model of impaired vascular remodeling. Atherosclerosis 2018;279:88–90. [DOI] [PubMed] [Google Scholar]
- 24. Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM.. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res 2011;109:697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanchis P, Ho CY, Liu Y, Beltran LE, Ahmad S, Jacob AP, Furmanik M, Laycock J, Long DA, Shroff R, Shanahan CM.. Arterial “inflammaging” drives vascular calcification in children on dialysis. Kidney Int 2019;95:958–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watson A, Nong Z, Yin H, O'Neil C, Fox S, Balint B, Guo L, Leo O, Chu MWA, Gros R, Pickering JG.. Nicotinamide phosphoribosyltransferase in smooth muscle cells maintains genome integrity, resists aortic medial degeneration, and is suppressed in human thoracic aortic aneurysm disease. Circ Res 2017;120:1889–1902. [DOI] [PubMed] [Google Scholar]
- 27. Barrett HE, Cunnane EM, Hidayat H, O'Brien JM, Moloney MA, Kavanagh EG, Walsh MT.. On the influence of wall calcification and intraluminal thrombus on prediction of abdominal aortic aneurysm rupture. J Vasc Surg 2018;67:1234–1246. e2. [DOI] [PubMed] [Google Scholar]
- 28. Yang C-J, Tsai S-H, Wang J-C, Chang W-C, Lin C-Y, Tang Z-C, Hsu H-H.. Association between acute aortic dissection and the distribution of aortic calcification. PLoS One 2019;14:e0219461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chowdhury MM, Zieliński LP, Sun JJ, Lambracos S, Boyle JR, Harrison SC, Rudd JHF, Coughlin PA.. Editor’s choice - calcification of thoracic and abdominal aneurysms is associated with mortality and morbidity. Eur J Vasc Endovasc Surg 2018;55:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Forsythe RO, Dweck MR, McBride OMB, Vesey AT, Semple SI, Shah ASV, Adamson PD, Wallace WA, Kaczynski J, Ho W, van Beek EJR, Gray CD, Fletcher A, Lucatelli C, Marin A, Burns P, Tambyraja A, Chalmers RTA, Weir G, Mitchard N, Tavares A, Robson JMJ, Newby DE.. 18F-sodium fluoride uptake in abdominal aortic aneurysms: the SoFIA3 study. J Am Coll Cardiol 2018;71:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li RX, Luo J, Balaram SK, Chaudhry FA, Shahmirzadi D, Konofagou EE.. Pulse wave imaging in normal, hypertensive and aneurysmal human aortas in vivo: a feasibility study. Phys Med Biol 2013;58:4549–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andreucci M, Provenzano M, Faga T, Michael A, Patella G, Mastroroberto P, Serraino GF, Bracale UM, Ielapi N, Serra R.. Aortic aneurysms, chronic kidney disease and metalloproteinases. Biomolecules 2021;11:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takagi H, Manabe H, Kawai N, Goto S-N, Umemoto T.. Circulating matrix metalloproteinase-9 concentrations and abdominal aortic aneurysm presence: a meta-analysis. Interact Cardiovasc Thorac Surg 2009;9:437–440. [DOI] [PubMed] [Google Scholar]
- 34. Provenzano M, Andreucci M, De Nicola L, Garofalo C, Battaglia Y, Borrelli S, Gagliardi I, Faga T, Michael A, Mastroroberto P, Serraino GF, Licastro N, Ielapi N, Serra R.. The role of prognostic and predictive biomarkers for assessing cardiovascular risk in chronic kidney disease patients. Biomed Res Int 2020;2020:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Provenzano M, Andreucci M, Garofalo C, Faga T, Michael A, Ielapi N, Grande R, Sapienza P, de Franciscis S, Mastroroberto P, Serra R.. The association of matrix metalloproteinases with chronic kidney disease and peripheral vascular disease: a light at the end of the tunnel? Biomolecules 2020;10:E154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Mast Q, Beutler JJ.. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens 2009;27:1333–1340. [DOI] [PubMed] [Google Scholar]
- 37. Studzińska D, Rudel B, Polok K, Lewandowski K, Studziński K, Gajdosz A, Oo A, Szczeklik M, Zaczek M, Zaniewski M, Szczeklik W.. Infrarenal versus suprarenal abdominal aortic aneurysms: comparison of associated aneurysms and renal artery stenosis. Ann Vasc Surg 2019;58:248–254. e1. [DOI] [PubMed] [Google Scholar]
- 38. Quill DS, Colgan MP, Sumner DS.. Ultrasonic screening for the detection of abdominal aortic aneurysms. Surg Clin North Am 1989;69:713–720. [DOI] [PubMed] [Google Scholar]
- 39. Lindholt JS, Vammen S, Juul S, Henneberg EW, Fasting H.. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 1999;17:472–475. [DOI] [PubMed] [Google Scholar]
- 40. Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, van Herwaarden JA, Holt PJE, van Keulen JW, Rantner B, Schlösser FJV, Setacci F, Ricco J-B; European Society for Vascular Surgery . Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg 2011;41 Suppl 1:S1–S58. [DOI] [PubMed] [Google Scholar]
- 41. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schermerhorn ML, Starnes BW.. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg 2018;67:2–77.e2. [DOI] [PubMed] [Google Scholar]
- 42. Wilmink ABM, Forshaw M, Quick CRG, Hubbard CS, Day NE.. Accuracy of serial screening for abdominal aortic aneurysms by ultrasound. J Med Screen 2002;9:125–127. [DOI] [PubMed] [Google Scholar]
- 43. Davenport MS, Perazella MA, Yee J, Dillman JR, Fine D, McDonald RJ, Rodby RA, Wang CL, Weinreb JC.. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology 2020;294:660–668. [DOI] [PubMed] [Google Scholar]
- 44. Cheng EL, Hong Q, Yong E, Chandrasekar S, Tan GWL, Lo ZJ.. Validating the use of contrast-induced nephropathy prediction models in endovascular aneurysm repairs. J Vasc Surg 2020;71:1546–1553. [DOI] [PubMed] [Google Scholar]
- 45. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G.. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 46. Chrysochou C, Power A, Shurrab AE, Husain S, Moser S, Lay J, Salama AD, Kalra PA.. Low risk for nephrogenic systemic fibrosis in nondialysis patients who have chronic kidney disease and are investigated with gadolinium-enhanced magnetic resonance imaging. Clin J Am Soc Nephrol 2010;5:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. American College of Radiology Manual on Contrast Media 2021 . https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. (10 April 2021, date last accessed).
- 48. Mathur M, Jones JR, Weinreb JC.. Gadolinium deposition and nephrogenic systemic fibrosis: a radiologist’s primer. Radiographics 2020;40:153–162. [DOI] [PubMed] [Google Scholar]
- 49. Woolen SA, Shankar PR, Gagnier JJ, MacEachern MP, Singer L, Davenport MS.. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: a systematic review and meta-analysis. JAMA Intern Med 2020;180:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, Dick F, van Herwaarden J, Karkos C, Koelemay M, Kölbel T, Loftus I, Mani K, Melissano G, Powell J, Szeberin Z, de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Kolh P, Lindholt JS, de Vega M, Vermassen F Document Reviewers Null Björck M, Cheng S, Dalman R, Davidovic L, Donas K, Earnshaw J, Eckstein H-H, Golledge J, Haulon S, Mastracci T, Naylor R, Ricco J-B, Verhagen H.. Editor’s Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg 2019;57:8–93. [DOI] [PubMed] [Google Scholar]
- 51. Powell JT, Brown LC, Forbes JF, Fowkes FGR, Greenhalgh RM, Ruckley CV, Thompson SG.. Final 12-year follow-up of surgery versus surveillance in the UK Small Aneurysm Trial. Br J Surg 2007;94:702–708. [DOI] [PubMed] [Google Scholar]
- 52. Crawford ES, Beckett WC, Greer MS.. Juxtarenal infrarenal abdominal aortic aneurysm. Special diagnostic and therapeutic considerations. Ann Surg 1986;203:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saratzis A, Joshi S, Benson RA, Bosanquet D, Dattani N, Batchelder A, Fisher O, Ioannidou E, Bown MJ, Imray CH; VERN Collaborators . Acute kidney injury (AKI) in aortic intervention: findings from the midlands aortic renal injury (MARI) cohort study. Eur J Vasc Endovasc Surg 2020;59:899–909. [DOI] [PubMed] [Google Scholar]
- 54. Dariane C, Coscas R, Boulitrop C, Javerliat I, Vilaine E, Goeau-Brissonniere O, Coggia M, Massy ZA.. Acute kidney injury after open repair of intact abdominal aortic aneurysms. Ann Vasc Surg 2017;39:294–300. [DOI] [PubMed] [Google Scholar]
- 55. Martin-Gonzalez T, Mastracci T, Carrell T, Constantinou J, Dias N, Katsargyris A, Modarai B, Resch T, Verhoeven E, Haulon S.. Mid-term outcomes of renal branches versus renal fenestrations for thoraco-abdominal aneurysm repair. Eur J Vasc Endovasc Surg 2016;52:141–148. [DOI] [PubMed] [Google Scholar]
- 56. Nadim MK, Forni LG, Bihorac A, Hobson C, Koyner JL, Shaw A, Arnaoutakis GJ, Ding X, Engelman DT, Gasparovic H, Gasparovic V, Herzog CA, Kashani K, Katz N, Liu KD, Mehta RL, Ostermann M, Pannu N, Pickkers P, Price S, Ricci Z, Rich JB, Sajja LR, Weaver FA, Zarbock A, Ronco C, Kellum JA.. Cardiac and vascular surgery-associated acute kidney injury: the 20th International Consensus Conference of the ADQI (Acute Disease Quality Initiative) Group. J Am Heart Assoc 2018;7:e008834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jhaveri KD, Saratzis AN, Wanchoo R, Sarafidis PA.. Endovascular aneurysm repair (EVAR)- and transcatheter aortic valve replacement (TAVR)-associated acute kidney injury. Kidney Int 2017;91:1312–1323. [DOI] [PubMed] [Google Scholar]
- 58. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2Suppl 1:1–138. [Google Scholar]
- 59. Pirgakis KM, Makris K, Dalainas I, Lazaris AM, Maltezos CK, Liapis CD.. Urinary cystatin C as an early biomarker of acute kidney injury after open and endovascular abdominal aortic aneurysm repair. Ann Vasc Surg 2014;28:1649–1658. [DOI] [PubMed] [Google Scholar]
- 60. Ueta K, Watanabe M, Iguchi N, Uchiyama A, Shirakawa Y, Kuratani T, Sawa Y, Fujino Y.. Early prediction of acute kidney injury biomarkers after endovascular stent graft repair of aortic aneurysm: a prospective observational study. J Intensive Care 2014;2:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pisimisis GT, Bechara CF, Barshes NR, Lin PH, Lai WS, Kougias P.. Risk factors and impact of proximal fixation on acute and chronic renal dysfunction after endovascular aortic aneurysm repair using glomerular filtration rate criteria. Ann Vasc Surg 2013;27:16–22. [DOI] [PubMed] [Google Scholar]
- 62. Saratzis A, Melas N, Mahmood A, Sarafidis P.. Incidence of acute kidney injury (AKI) after endovascular abdominal aortic aneurysm repair (EVAR) and impact on outcome. Eur J Vasc Endovasc Surg 2015;49:534–540. [DOI] [PubMed] [Google Scholar]
- 63. Saratzis A, Harrison S, Barratt J, Sayers RD, Sarafidis PA, Bown MJ.. Intervention associated acute kidney injury and long-term cardiovascular outcomes. Am J Nephrol 2015;42:285–294. [DOI] [PubMed] [Google Scholar]
- 64. Saratzis A, Nduwayo S, Sarafidis P, Sayers RD, Bown MJ.. Renal function is the main predictor of acute kidney injury after endovascular abdominal aortic aneurysm repair. Ann Vasc Surg 2016;31:52–59. [DOI] [PubMed] [Google Scholar]
- 65. Obata Y, Kamijo-Ikemori A, Ichikawa D, Sugaya T, Kimura K, Shibagaki Y, Tateda T.. Clinical usefulness of urinary liver-type fatty-acid-binding protein as a perioperative marker of acute kidney injury in patients undergoing endovascular or open-abdominal aortic aneurysm repair. J Anesth 2016;30:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee J, Park K-M, Jung S, Cho W, Hong KC, Jeon YS, Cho SG, Lee JB.. Occurrences and results of acute kidney injury after endovascular aortic abdominal repair? Vasc Specialist Int 2017;33:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Saratzis A, Chiocchia V, Jiffry A, Hassanali N, Singh S, Imray CH, Bown MJ, Mahmood A.. HYDration and bicarbonate to prevent acute renal injury after endovascular aneurysm repair with suprarenal fixation: pilot/feasibility randomised controlled study (HYDRA pilot trial). Eur J Vasc Endovasc Surg 2018;55:648–656. [DOI] [PubMed] [Google Scholar]
- 68. Zabrocki L, Marquardt F, Albrecht K, Herget-Rosenthal S.. Acute kidney injury after abdominal aortic aneurysm repair: current epidemiology and potential prevention. Int Urol Nephrol 2018;50:331–337. [DOI] [PubMed] [Google Scholar]
- 69. Saratzis AN, Bath MF, Harrison SC, Sayers RD, Bown MJ.. Impact of fenestrated endovascular abdominal aortic aneurysm repair on renal function. J Endovasc Ther 2015;22:889–896. [DOI] [PubMed] [Google Scholar]
- 70. Sailer AM, Nelemans PJ, van Berlo C, Yazar O, de Haan MW, Fleischmann D, Schurink GW.. Endovascular treatment of complex aortic aneurysms: prevalence of acute kidney injury and effect on long-term renal function. Eur Radiol 2016;26:1613–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ducasse E, Caradu C, Elicagaray A, Berard X, Midy D, Stecken L.. Early impact on renal parenchymal vascularization of chimney grafts versus fenestrated grafts. Eur J Vasc Endovasc Surg 2016;51:647–655. [DOI] [PubMed] [Google Scholar]
- 72. Tran K, Fajardo A, Ullery BW, Goltz C, Lee JT.. Renal function changes after fenestrated endovascular aneurysm repair. J Vasc Surg 2016;64:273–280. [DOI] [PubMed] [Google Scholar]
- 73. Tinelli G, Crea MA, de Waure C, Di Tanna GL, Becquemin J-P, Sobocinski J, Snider F, Haulon S.. A propensity-matched comparison of fenestrated endovascular aneurysm repair and open surgical repair of pararenal and paravisceral aortic aneurysms. J Vasc Surg 2018;68:659–668. [DOI] [PubMed] [Google Scholar]
- 74. Wang SK, Lemmon GW, Gupta AK, Dalsing MC, Sawchuk AP, Motaganahalli RL, Murphy MP, Fajardo A.. Fenestrated endovascular aneurysm repair-induced acute kidney injury does not result in chronic renal dysfunction. J Vasc Surg 2019;69:1679–1684. [DOI] [PubMed] [Google Scholar]
- 75. Khoury MK, Timaran DE, Soto-Gonzalez M, Timaran CH.. Fenestrated-branched endovascular aortic repair in patients with chronic kidney disease. J Vasc Surg 2020;72:66–72. [DOI] [PubMed] [Google Scholar]
- 76. Reis PV, Morgado M, Valdoleiros I, Neto MD, Mourão J.. Complications of endovascular aneurysm repair: mortality, myocardial infarction and acute kidney injury. Turk J Anaesthesiol Reanim 2018;46:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fortrie G, de Geus HRH, Betjes MGH.. The aftermath of acute kidney injury: a narrative review of long-term mortality and renal function. Crit Care 2019;23:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Al Adas Z, Shepard AD, Nypaver TJ, Weaver MR, Maatman T, Yessayan LT, Balraj P, Kabbani LS.. Long-term decline in renal function is more significant after endovascular repair of infrarenal abdominal aortic aneurysms. J Vasc Surg 2018;68:739–748. [DOI] [PubMed] [Google Scholar]
- 79. Calderbank T, Bown M, Saratzis A.. The impact of suprarenal fixation on renal function following endovascular abdominal aortic aneurysm repair: meta-analysis based on estimated glomerular filtration rate. Eur J Vasc Endovasc Surg 2018;56:497–506. [DOI] [PubMed] [Google Scholar]
- 80. Saratzis A, Bown MJ.. Renal injury after endovascular aneurysm repair: an overlooked entity. Eur J Vasc Endovasc Surg 2016;51:325–326. [DOI] [PubMed] [Google Scholar]
- 81. National Clinical Guideline Centre (UK) . Acute Kidney Injury: Prevention, Detection and Management up to the Point of Renal Replacement Therapy [Internet]. London: Royal College of Physicians (UK); 2013. [PubMed] [Google Scholar]
- 82. Moore NN, Lapsley M, Norden AG, Firth JD, Gaunt ME, Varty K, Boyle JR.. Does N-acetylcysteine prevent contrast-induced nephropathy during endovascular AAA repair? A randomized controlled pilot study. J Endovasc Ther 2006;13:660–666. [DOI] [PubMed] [Google Scholar]
- 83. Walsh SR, Boyle JR, Tang TY, Sadat U, Cooper DG, Lapsley M, Norden AG, Varty K, Hayes PD, Gaunt ME.. Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: a randomized controlled trial. J Endovasc Ther 2009;16:680–689. [DOI] [PubMed] [Google Scholar]
- 84. Allie DE, Lirtzman MD, Wyatt CH, Keller VA, Mitran EV, Hebert CJ, Patlola R, Veerina KK, Walker CM.. Targeted renal therapy and contrast-induced nephropathy during endovascular abdominal aortic aneurysm repair: results of a feasibility pilot trial. J Endovasc Ther 2007;14:520–527. [DOI] [PubMed] [Google Scholar]
- 85. Brulotte V, Leblond FA, Elkouri S, Thérasse E, Pichette V, Beaulieu P.. Bicarbonates for the prevention of postoperative renal failure in endovascular aortic aneurysm repair: a randomized pilot trial. Anesthesiol Res Pract 2013;2013:467326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dennis JM, Witting PK.. Protective role for antioxidants in acute kidney disease. Nutrients 2017;9: 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Go MR, Barbato JE, Rhee RY, Makaroun MS.. What is the clinical utility of a 6-month computed tomography in the follow-up of endovascular aneurysm repair patients? J Vasc Surg 2008;47:1181–1186; discussion 1186–1187. [DOI] [PubMed] [Google Scholar]
- 88. Cantisani V, Ricci P, Grazhdani H, Napoli A, Fanelli F, Catalano C, Galati G, D'Andrea V, Biancari F, Passariello R.. Prospective comparative analysis of colour-Doppler ultrasound, contrast-enhanced ultrasound, computed tomography and magnetic resonance in detecting endoleak after endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2011;41:186–192. [DOI] [PubMed] [Google Scholar]
- 89. Saratzis A, Sarafidis P, Melas N, Hunter JP, Saratzis N, Kiskinis D, Kitas GD.. Suprarenal graft fixation in endovascular abdominal aortic aneurysm repair is associated with a decrease in renal function. J Vasc Surg 2012;56:594–600. [DOI] [PubMed] [Google Scholar]
- 90. Saratzis A, Sarafidis P, Melas N, Khaira H.. Comparison of the impact of open and endovascular abdominal aortic aneurysm repair on renal function. J Vasc Surg 2014;60:597–603. [DOI] [PubMed] [Google Scholar]
- 91. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, Society for Vascular Medicine . 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27–e129. [DOI] [PubMed] [Google Scholar]
- 92. Hoogmoed RC, Patel HJ, Kim KM, Williams DM, Deeb GM, Yang B.. Acute kidney injury in acute type B aortic dissection: outcomes over 20 years. Ann Thorac Surg 2019;107:486–492. [DOI] [PubMed] [Google Scholar]
- 93. Tolenaar JL, Froehlich W, Jonker FHW, Upchurch GR, Rampoldi V, Tsai TT, Bossone E, Evangelista A, O’Gara P, Pape L, Montgomery D, Isselbacher EM, Nienaber CA, Eagle KA, Trimarchi S.. Predicting in-hospital mortality in acute type B aortic dissection: evidence from International Registry of Acute Aortic Dissection. Circulation 2014;130:S45–50. [DOI] [PubMed] [Google Scholar]
- 94. Reutersberg B, Salvermoser M, Trenner M, Geisbüsch S, Zimmermann A, Eckstein H-H, Kuehnl A.. Hospital incidence and in-hospital mortality of surgically and interventionally treated aortic dissections: secondary data analysis of the Nationwide German Diagnosis-Related Group Statistics From 2006 to 2014. J Am Heart Assoc 2019;8:e011402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ogami T, Zimmermann E, Zhu RC, Zhao Y, Ning Y, Kurlansky P, Stevens JS, Avgerinos DV, Patel VI, Takayama H.. Proximal aortic repair in dialysis patients: a national database analysis. J Thorac Cardiovasc Surg 2021;S0022-5223:00404–00409. [DOI] [PubMed] [Google Scholar]
- 96. Kaji S. Acute medical management of aortic dissection. Gen Thorac Cardiovasc Surg 2019;67:203–207. [DOI] [PubMed] [Google Scholar]
- 97. Takahashi T, Hasegawa T, Hirata N, Endo A, Yamasaki Y, Ashida K, Kabeya Y, Nakagawa S.. Impact of acute kidney injury on in-hospital outcomes in patients with DeBakey type III acute aortic dissection. Am J Cardiol 2014;113:1904–1910. [DOI] [PubMed] [Google Scholar]
- 98. Li L, Wang M, Li J, Guan X, Xin P, Wang X, Liu Y, Li H, Jiang W, Gong M, Zhang H.. Short term prognosis of renal artery stenosis secondary to acute type B aortic dissection with TEVAR. Front Cardiovasc Med 2021;8:658952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Joh JH, Park Y-Y, Cho S-S, Park H-C.. National trends for open and endovascular repair of aneurysms in Korea: 2004-2013. Exp Ther Med 2016;12:3333–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Qureshi MI, Davies AH.. Endovascular aneurysm repair in the elderly: first do no harm. Vascular 2018;26:113–114. [DOI] [PubMed] [Google Scholar]
- 101. Patel R, Powell JT, Sweeting MJ, Epstein DM, Barrett JK, Greenhalgh RM.. The UK EndoVascular Aneurysm Repair (EVAR) randomised controlled trials: long-term follow-up and cost-effectiveness analysis. Health Technol Assess 2018;22:1–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. De Rango P, Simonte G, Manzone A, Farchioni L, Cieri E, Verzini F, Parlani G, Isernia G, Lenti M.. Mortality risk for ruptured abdominal aortic aneurysm in women. Ann Vasc Surg 2017;39:143–151. [DOI] [PubMed] [Google Scholar]
- 103. Sidloff DA, Saratzis A, Sweeting MJ, Michaels J, Powell JT, Thompson SG, Bown MJ.. Sex differences in mortality after abdominal aortic aneurysm repair in the UK. Br J Surg 2017;104:1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lo RC, Bensley RP, Hamdan AD, Wyers M, Adams JE, Schermerhorn ML; Vascular Study Group of New England . Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg 2013;57:1261–1268. 1268.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a conference report and no original data are available.