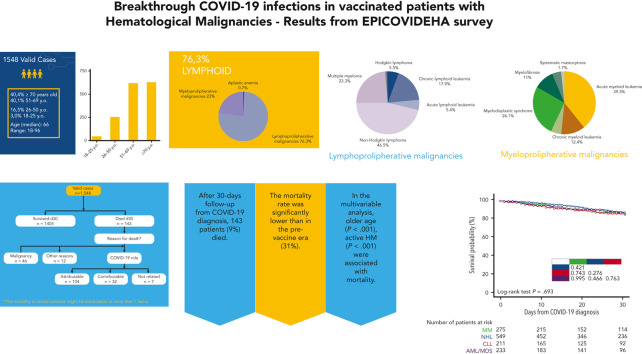
Visual Abstract
Abstract
Limited data are available on breakthrough COVID-19 in patients with hematologic malignancy (HM) after anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination. Adult patients with HM, ≥1 dose of anti-SARS-CoV-2 vaccine, and breakthrough COVID-19 between January 2021 and March 2022 were analyzed. A total of 1548 cases were included, mainly lymphoid malignancies (1181 cases, 76%). After viral sequencing in 753 cases (49%), the Omicron variant was prevalent (517, 68.7%). Most of the patients received ≤2 vaccine doses before COVID-19 (1419, 91%), mostly mRNA-based (1377, 89%). Overall, 906 patients (59%) received COVID-19-specific treatment. After 30-day follow-up from COVID-19 diagnosis, 143 patients (9%) died. The mortality rate in patients with the Omicron variant was 7.9%, comparable to other variants, with a significantly lower 30-day mortality rate than in the prevaccine era (31%). In the univariable analysis, older age (P < .001), active HM (P < .001), and severe and critical COVID-19 (P = .007 and P < .001, respectively) were associated with mortality. Conversely, patients receiving monoclonal antibodies, even for severe or critical COVID-19, had a lower mortality rate (P < .001). In the multivariable model, older age, active disease, critical COVID-19, and 2-3 comorbidities were correlated with a higher mortality, whereas monoclonal antibody administration, alone (P < .001) or combined with antivirals (P = .009), was protective. Although mortality is significantly lower than in the prevaccination era, breakthrough COVID-19 in HM is still associated with considerable mortality. Death rate was lower in patients who received monoclonal antibodies, alone or in combination with antivirals.
Pagano and colleagues provide new insight into outcomes for vaccinated patients with blood cancers in the Omicron variant era of the current COVID-19 pandemic. Based on registry data, the rate of mortality with breakthrough COVID-19 infection postvaccination is 9%. Multivariable analysis indicates that patients who have received monoclonal antibodies, alone or with antivirals, have better clinical outcomes. These and other data confirm that COVID-19 remains a threat to vulnerable patients and that both vaccination and treatment strategies are necessary as new variants emerge.
Introduction
Coronavirus disease 2019 (COVID-19) is a life-threatening infection in patients with hematologic malignancies (HM), associated with severe clinical presentation and high risk of death.1, 2, 3 In April 2020, the European Hematology Association’s Specialized Working Group, Infections in Hematology, opened the EPICOVIDEHA (Epidemiology of COVID-19 Infection in Patients with Hematological Malignancies: European Haematology Association) registry to collect data on all adult patients with HM who developed COVID-19. It aimed to describe the epidemiology and risk factors, and reported a mortality rate of 31.2% among 3801 patients.4 In December 2020, nearly 1 year after the first described COVID-19 case, vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were approved and became available first to patients at high risk, including patients with HM.5, 6, 7, 8 The recently published recommendations from the European Conference of Infections in Leukemia (ECIL-9) identify the critical role of messenger RNA (mRNA)-based vaccines in the fight against COVID-19 and recommend their use in HM, although they may have more limited efficacy among severely immunocompromised patients.9
We collected data on adults with HM who developed breakthrough COVID-19, to assess the vaccine efficacy and the potential role of new emergent treatments against SARS-CoV-2. Our preliminary data, regarding the first 113 patients included, showed a significant decrease in the overall mortality rate in the postvaccination era (12.4%), which was, however, still remarkably higher than the rate observed in the overall population.10 To date, few reports have been published on the severity and outcomes of breakthrough COVID-19 in patients with cancer in general11 , 12 and HM specifically,13 all showing high rates of severe clinical presentation, hospitalization, and death among these patients. This suggests that HM require close monitoring and increased medical attention when COVID-19 is diagnosed, regardless of previous anti-SARS-CoV-2 vaccine administration.
In this study, we analyzed the epidemiology and outcome of breakthrough COVID-19 in a large cohort of patients with HMs and evaluated anti-SARS-CoV-2 treatment received by these patients.
Methods
Study design, patients, and procedures
From 1 January 2021 to 10 March 2022, participating institutions documented episodes of COVID-19 in patients with HM that received anti-SARS-CoV-2 vaccination. Our analysis comprised data from the EPICOVIDEHA registry. EPICOVIDEHA (www.clinicaltrials.gov; National Clinical Trials identifier NCT04733729) is an international open web-based registry for patients with HMs infected with SARS-CoV-2.14 The EPICOVIDEHA registry was approved by the local ethics committee of the Fondazione Policlinico Universitario Agostino Gemelli, RCCS, Università Cattolica del Sacro Cuore of Rome, Italy (Study ID: 3226). When applicable, the respective local ethics committee of each participating institution had approved the project. EPICOVIDEHA methods have been described elsewhere.4 , 14 The electronic case report form is accessible online at www.clinicalsurveys.net (EFS Summer 2021, Tivian, Cologne, Germany). Each patient that had been documented was reviewed and validated by infectious diseases and hematology experts from the coordination team. Inclusion criteria were: (1) active HM within the last 5 years before COVID-19 diagnosis, (2) aged ≥18 years, (3) laboratory-based diagnosis of SARS-CoV-2 infection, and (4) last vaccine dose ≥15 days before PCR confirmed SARS-CoV-2 infection. Data on baseline conditions pre-COVID-19 (ie, age, sex, status of HM at COVID-19 diagnosis, COVID-19–predisposing factors), HM clinical management (ie, last HM treatment strategy), vaccine type, spike protein concentration at diagnosis of COVID-19, COVID-19 diagnosis and management (ie, reason for diagnostic test, symptoms at onset, hospital stay during infection, treatments received for infection), and outcome (ie, mortality, attributable mortality [assessed by the medical team in charge of the patient], and last day of follow-up) were collected. Status of HM at COVID-19 onset and last follow-up was defined as active (onset and refractory/resistant), stable disease, or controlled (complete and partial response) based on the reports from the respective participating institution.
Study objectives
The primary objective of this study was to assess the epidemiology and the outcome of HM affected by breakthrough COVID-19. Secondary objectives were: (1) to estimate the relative frequency of disease severity, graded according to international standards in our patient population;15 , 16 (2) to evaluate the relative frequency of intensive care unit (ICU) admission among participating patients; (3) to evaluate the overall case-fatality rate; (4) to explore the effect of cancer treatment phase (induction, consolidation, maintenance, palliative, or reinduction) on patient outcomes; (5) to explore the effect of vaccine doses administered on patient outcomes; and (6) to explore the effect of COVID-19 treatment on patient outcomes. Moreover, data collected were compared with data reported in our previously published study performed in the prevaccine era by using the same registry.4
Sample size and statistical analysis
No a priori sample size calculation was performed for this analysis. Categorical variables are presented with frequencies and percentages, and continuous variables with median, interquartile range (IQR), and absolute range. A univariable Cox regression model was performed with variables suspected to play a role in the mortality of patients with HM with COVID-19. Variables with a P value ≤.1 were considered for multivariable analysis. A multivariable Cox regression model was calculated with the Wald backward method. Mortality was analyzed by using Kaplan–Meier survival plots. A log-rank test was used to compare the survival probability of the patients included in the different models. A P value ≤.05 was considered statistically significant. No a priori sample size calculation was done for this exploratory study. SPSS version 25.0 was employed for statistical analyses (SPSS, IBM Corp, Chicago, IL). Patients with missing data in essential fields (ie, HM, chemotherapeutic program, vaccination status, COVID-19 management, or survival status) were considered as “not valid” and excluded from the final analysis. Among the valid cases, if a value in a specific variable was missing or unknown, it is indicated as such in the descriptive analysis. Patients with missing data in a certain variable were excluded from regression analyses in case that variable was included into such analyses.
Results
Study population
A total of 94 centers in 26 countries, mainly from Europe, participated and registered 1583 cases. A list of enrolled cases from each participating country is available in the supplemental material (supplemental Figures 1 and 2A, available on the Blood website). Out of these 1583 cases, 35 were excluded because COVID-19 was diagnosed within 14 days from the first vaccine dose. Clinical characteristics of 1548 evaluable cases are reported in Table 1 . Lymphoid malignancies were the largest subgroup, accounting for 1181 cases (76.3%); the most frequently reported diagnosis was non-Hodgkin lymphoma (NHL, 549 cases). Among myeloid malignancies, the most frequent diagnosis was acute myeloid leukemia (AML, 140 cases). We found a significantly different distribution of lymphoid/myeloid malignancies from that reported in the prevaccination era (prevaccination lymphoid malignancy cases, 67.3%, vs postvaccination, 76.3%, P < .001). At the time of COVID-19 diagnosis, most patients had a controlled malignancy (n = 821, 53%), 322 (20.8%) a stable disease, and the remaining 365 (23.6%) an active disease, with 185 cases registered at HM onset. The most frequently reported last HM treatment was immuno-chemotherapy or immunotherapy alone (n = 708, 42%), followed by targeted therapies (n = 311, 20.1%) and conventional chemotherapy (n = 234, 15.1%); 92 patients (5.9%) had received hematopoietic stem cell transplantation within 6 months before COVID-19 (allogeneic 76; autologous, 16) and 8 had chimeric antigen receptor T-cell therapy. Most patients presented at least 1 comorbidity (60.7%) and 180 (11.6%) had a history of smoking; a complete list of comorbidities and associated clinical outcomes is available in the supplemental material (supplemental Table 1).
Table 1.
Clinical characteristics of 1548 vaccinated patients with HM who developed COVID-19
| n | % | |
|---|---|---|
| Sex | ||
| Female/male | 661/887 | 42.7/57.3 |
| Age | ||
| Median, y (IQR) [range] | 66 (55-75) [18-96] | |
| <50/>50 y | 301/1247 | 19.5/80.5 |
| Comorbidities | ||
| None/1-2-3 comorbidities | 608/940 | 39.3/60.7 |
| Smoking history | 180 | 11.6 |
| Malignancy | ||
| Lymphoid malignancies | 1181 | 76.3 |
| Acute lymphoid leukemia | 64 | 4.1 |
| Chronic lymphoid leukemia | 211 | 13.6 |
| Hodgkin lymphoma | 65 | 4.2 |
| Non-Hodgkin lymphoma | 549 | 35.5 |
| Low grade | 289 | 18.7 |
| High grade | 260 | 16.8 |
| Multiple myeloma | 275 | 17.8 |
| Amyloid light-chain amyloidosis | 10 | 0.6 |
| Hairy cell leukemia | 7 | 0.5 |
| Myeloid malignancies | 356 | 23.0 |
| Acute myeloid leukemia | 140 | 9.0 |
| Chronic myeloid leukemia | 44 | 2.8 |
| Essential thrombocythemia | 18 | 1.2 |
| Myelodysplastic syndromes | 93 | 6.0 |
| Low-intermediate risk | 69 | 4.5 |
| High risk | 23 | 1.5 |
| Myelofibrosis | 39 | 2.5 |
| Polycythemia vera | 16 | 1.0 |
| Systemic mastocytosis | 6 | 0.4 |
| Aplastic anemia | 11 | 0.7 |
| Malignancy status before COVID-19 | ||
| Controlled disease | 821 | 53.0 |
| Complete remission | 524 | 33.9 |
| Partial remission | 297 | 19.2 |
| Stable disease | 322 | 20.8 |
| Active disease | 365 | 23.6 |
| Onset | 185 | 12.0 |
| Refractory/resistant | 180 | 11.6 |
| Unknown | 40 | 2.6 |
| Last malignancy treatment | ||
| Allo-HSCT | 76 | 4.9 |
| Auto-HSCT | 16 | 1 |
| CAR-T | 8 | 0.5 |
| Chemotherapy | ||
| Conventional chemotherapy | 234 | 15.1 |
| Demethylating agents | 80 | 5.2 |
| Immunotherapy | 146 | 5.7 |
| Immuno-chemotherapy | 562 | 36.3 |
| Targeted therapy | 311 | 20.1 |
| Supportive measures | 36 | 2.3 |
| No treatment | 136 | 8.8 |
| Vaccination | ||
| 1 dose | 129 | 8.3 |
| 2 doses (or J&J) | 770 | 49.7 |
| 3 doses | 639 | 41.3 |
| 4 doses | 10 | 0.6 |
| Type of vaccine | ||
| mRNA | 1377 | 89.0 |
| BioNTech/Pfizer | 1121 | 72.4 |
| Moderna COVE | 256 | 16.5 |
| Vector-based | 133 | 8.6 |
| AstraZeneca Oxford | 99 | 6.4 |
| Sputnik | 13 | 0.8 |
| J&J (Janssen) | 21 | 1.4 |
| Inactivated | 38 | 2.5 |
| CoronaVac/Sinovac | 21 | 1.4 |
| Sinopharm | 17 | 1.1 |
| Spike protein dosage after vaccination∗ | ||
| No response | 135 | 8.7 |
| Weak response | 34 | 2.2 |
| Optimal response | 75 | 4.8 |
| Not tested | 1304 | 84.2 |
| COVID-19 infection | ||
| Wild type | 40 | 2.6 |
| Alpha | 34 | 2.2 |
| Beta | 1 | 0.1 |
| Delta | 161 | 10.4 |
| Omicron | 517 | 33.4 |
| Not tested | 795 | 51.4 |
| Severity | ||
| Asymptomatic | 283 | 18.3 |
| Mild infection | 604 | 39.0 |
| Severe infection | 509 | 32.9 |
| Critical infection | 152 | 9.8 |
| Symptomatology at onset | ||
| Asymptomatic | 306 | 19.8 |
| Pulmonary | 528 | 34.1 |
| Pulmonary + extrapulmonary | 400 | 25.8 |
| Extrapulmonary | 314 | 20.3 |
| Stay during COVID-19 | ||
| Hospital | 823 | 53.2 |
| ICU | 152 | 9.8 |
| Home | 800 | 51.7 |
CAR-T, chimeric antigen receptor T cells; HSCT, hematopoietic stem cell transplantation.
Referring to the World Health Organization international standards, binding antibody units per milliliter (https://www.who.int/news-room/feature-stories/detail/standardization-of-vaccines-for-coronavirusdisease-covid-19).
COVID-19 severity, variants, and anti-SARS-CoV-2 spike proteins
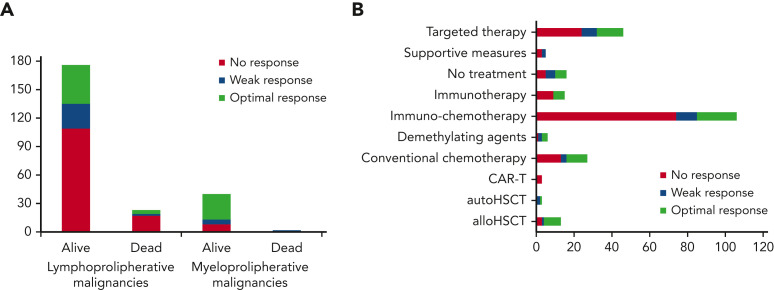
COVID-19 was mild, severe, or critical in 39%, 32.9%, and 9.8% of cases, respectively. Of the 1548 patients, 283 (18.3%) were asymptomatic and in most of them the diagnosis was made in screening programs (Table 1). We found a significantly lower rate of severe or critical cases compared with what we reported in the prevaccination era (prevaccination, 2425/3801, 63.8%, vs postvaccination, 661/1545, 42.7%; P < .001). Overall, 823 (53.2%) patients required hospitalization and among them 152 (18.1%) required admission to ICUs. The hospitalization and ICU admission rate was significantly lower than reported in the prevaccination era (53.2% vs 73%; P < .001% and 9.8% vs 18.1%; P < .001, respectively). The percentage of asymptomatic cases was 18.3% (283/1548), similar to that reported in our previous publication with data from the prevaccine era (17.8%, 675/3801).4 Viral genomes were studied in 753 cases (48.6%), with the different Omicron variant the most frequently detected viral strain (517/753, 68.7%). Most patients received 2 or 3 anti-SARS-CoV-2 vaccine doses (91%), mostly with mRNA-based technology (89%); only a few patients (8.6%) received a vector-based vaccine and a minority of them an inactivated vaccine (Table 1; supplemental Figure 2B-D). Anti-SARS-CoV-2 spike protein immunoglobulin G (IgG) levels were analyzed in 244 (15.8%) fully vaccinated patients, 2 to 4 weeks after the last vaccine dose; among these patients, 109 (44.7%) presented an antibody response (optimal, 75, 30.7%; weak, 34, 13.9%), whereas the remaining 135 (55.3%) were nonresponders. Most patients who did not have a serological response to vaccines were affected by lymphoid malignancies, as expected (126/135, 93.3%; Figure 1 ).
Figure 1.
Patient distribution by serological response after last COVID-19 vaccination before COVID-19 diagnosis. (A) By baseline malignancy; (B) by last treatment for HM immediately before COVID-19 diagnosis.
COVID-19 treatments and risk factors for mortality
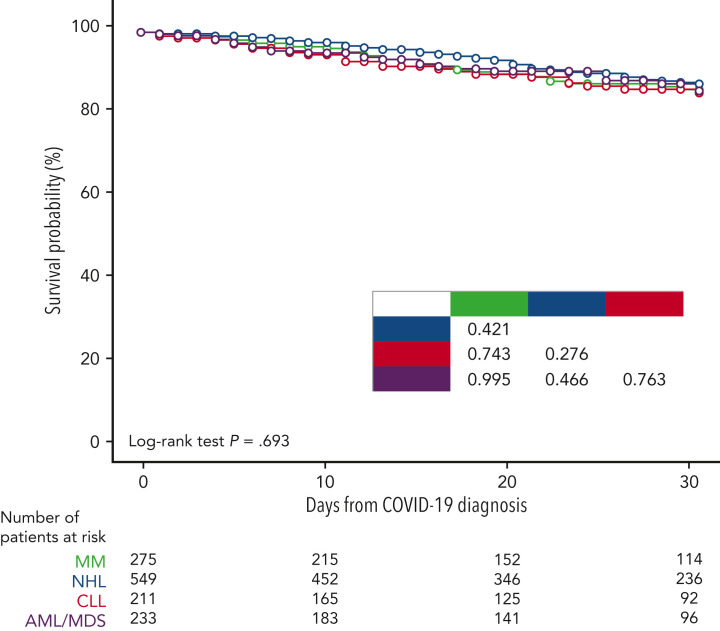
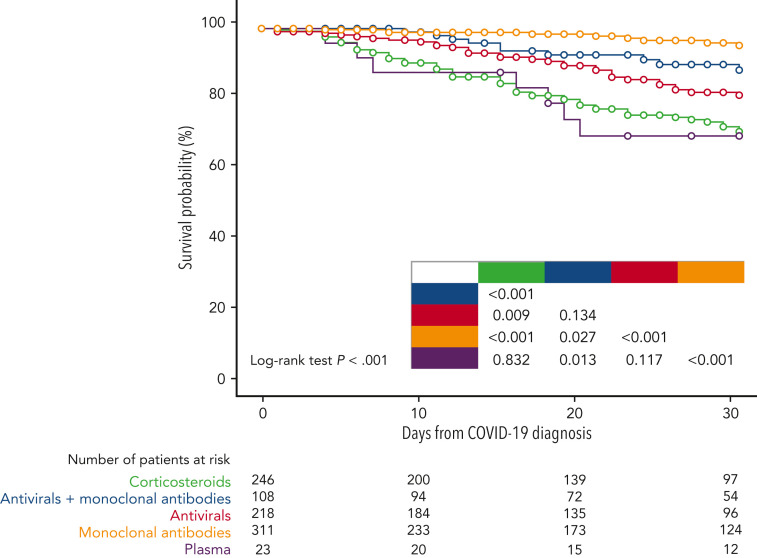
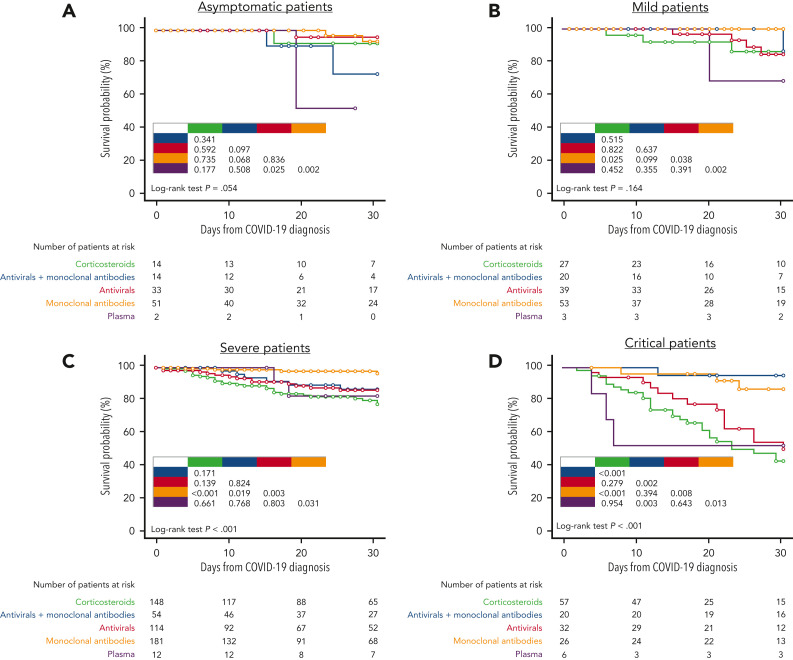
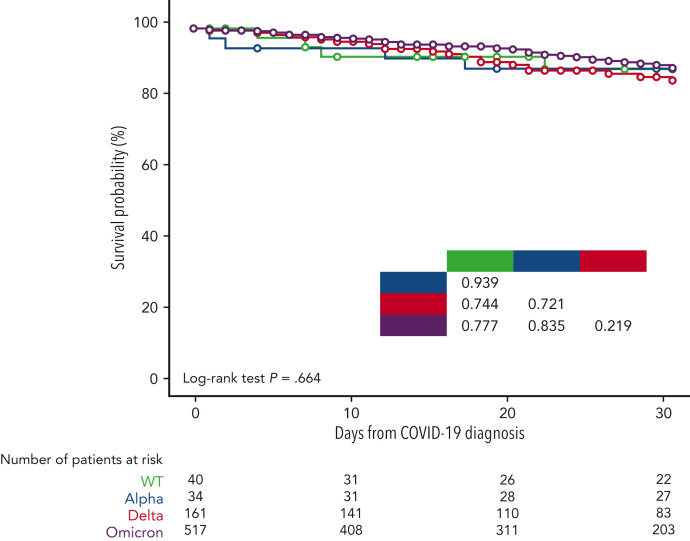
Overall, 906 patients (58.5%) received a specific treatment for COVID-19, whereas 642 (41.5%) were not treated or received symptomatic therapies (nonsteroidal anti-inflammatories, painkillers, antipyretics). Among patients who received a specific treatment for COVID-19, 311 (34.3%) were treated with monoclonal antibodies only, 246 (27.1%) with corticosteroids only, 218 (24.1%) with antivirals only, 108 (11.9%) with antiviral plus monoclonal antibodies, and the remaining 23 with convalescent plasma. Details on COVID-19 treatments and outcomes are displayed in the supplemental material (supplemental Table 2). Overall day-30 mortality (ie, from COVID-19 diagnosis) was 9.2% (143/1548); if we only consider patients who were symptomatic, the day-30 mortality rate was 0.3% (130/1265). The primary cause of death was COVID-19 in 97 patients (67.8%), a combination of COVID-19 and progressive HM in 39 cases (27.2%), and HM alone or combined with other reasons in the remaining 7 patients (4.8%). The mortality rate was significantly lower than that reported in the prevaccine era (prevaccine 31.2% vs postvaccine 9.2%; P < .001). Looking at 2 of the largest patient cohorts (ie, chronic lymphocytic leukemia and NHL) we evaluated the potential effect of chemotherapeutic treatment type on mortality rate. In patients with chronic lymphocytic leukemia, we did not observe any significant difference in terms of 30-day mortality rate among patients who had received immuno-chemotherapy (13.4%), immunotherapy alone (12.5%), or new targeted therapies (16.1%). On the contrary, in NHL we did observe a slightly higher mortality rate for patients recently treated with chimeric antigen receptor T cells (20%), compared with those treated with immuno-chemotherapy (8%), immunotherapy alone (14.3%), or targeted therapies (9.5%). Patient outcomes according to clinical characteristics, vaccine received, and specific treatments against SARS-CoV-2 are detailed in Table 2 . As shown in Figure 2 , we did not find any significant difference in terms of 30-day mortality rate among the different HM (P = .693), in contrast to that observed in the prevaccination era in which we reported a higher number of fatalities in patients with AML/myelodysplastic syndrome. In univariable analysis, the factors associated with a worse mortality rate were older age (P < .001), active HM disease (P < .001), and presence of 2 to 3 comorbidities (P < .001) (Table 3 ). Regarding age, patients aged <60 years showed a more favorable outcome (30-day mortality rate, 2.6%), compared with patients aged 60 to 69 years (7%), 70 to 79 years (14.8%), and ≥80 years (19.6%) (P < .001). Conversely, we observed a better clinical outcome for patients who received monoclonal antibodies (with or without antivirals; Figure 3 ). Analyzing the severity of COVID-19 presentation, a better clinical outcome was observed in patients treated with monoclonal antibodies alone for asymptomatic, mild, or severe disease and with monoclonal antibodies combined with antivirals in critical cases (Figure 4 ). We did not find differences in terms of outcome according to the number of vaccine doses received; however, a slightly better clinical outcome was evident among patients who received 3 to 4 doses vs 1 to 2 doses (P = .040, Table 3). We did not observe differences in survival when sorting patients according to viral strain detected (P = .664; Figure 5 ), or postvaccine antispike IgG levels (Table 2).
Table 2.
Outcome of vaccinated patients with HM who developed COVID-19
| Alive |
Deceased |
P value |
|||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Outcome at 30 d after COVID-19 diagnosis | |||||
| Alive | 1405 | 90.8 | |||
| Deceased | 143 | 9.2 | |||
| Reason for death | |||||
| COVID-19 | 97 | 67.8 | |||
| COVID-19 + HM | 39 | 27.2 | |||
| HM ± other reasons | 7 | 4.8 | |||
| Sex | |||||
| Female | 591 | 89.4 | 70 | 10.6 | ns |
| Male | 814 | 91.8 | 73 | 8.2 | |
| Age | |||||
| 18-25 y | 46 | 100.0 | 0 | 0.0 | <.001 |
| 26-50 y | 250 | 98.0 | 5 | 2.0 | |
| 51-69 y | 585 | 94.2 | 36 | 5.8 | |
| >70 y | 524 | 83.7 | 102 | 16.3 | |
| Comorbidities | |||||
| No comorbidities | 581 | 95.6 | 27 | 4.4 | <.001 |
| 1 comorbidity | 471 | 91.5 | 44 | 8.5 | |
| 2 comorbidities | 223 | 84.8 | 40 | 15.2 | |
| ≥3 comorbidities | 130 | 80.2 | 32 | 19.8 | |
| Smoker or ex-smoker | 158 | 87.8 | 22 | 12.2 | |
| Malignancies | |||||
| Lymphoid malignancies | 1070 | 92.8 | 111 | 7.2 | ns |
| Acute lymphoid leukemia | 62 | 96.9 | 2 | 3.1 | |
| Chronic lymphoid leukemia | 186 | 88.2 | 25 | 11.8 | |
| Hodgkin lymphoma | 63 | 96.9 | 2 | 3.1 | |
| Non-Hodgkin lymphoma | 497 | 90.5 | 52 | 9.5 | |
| Low grade | 261 | 90.3 | 28 | 9.7 | |
| High grade | 236 | 90.8 | 24 | 9.2 | |
| Multiple myeloma | 246 | 89.5 | 29 | 10.5 | |
| Amyloid light-chain amyloidosis | 10 | 100.0 | 0 | 0.0 | |
| Hairy cell leukemia | 6 | 85.7 | 1 | 14.3 | |
| Myeloid malignancies | 324 | 91.0 | 32 | 9.0 | |
| Acute myeloid leukemia | 127 | 90.7 | 13 | 9.3 | |
| Chronic myeloid leukemia | 43 | 97.7 | 1 | 2.3 | |
| Essential thrombocythemia | 18 | 100.0 | 0 | 0.0 | |
| Myelodysplastic syndromes | 81 | 87.1 | 12 | 12.9 | |
| Low-intermediate risk | 63 | 91.3 | 6 | 8.7 | |
| High risk | 18 | 78.3 | 5 | 21.7 | |
| Myelofibrosis | 34 | 87.2 | 5 | 12.8 | |
| Polycythemia vera | 15 | 93.8 | 1 | 6.3 | |
| Systemic mastocytosis | 6 | 100.0 | 0 | 0.0 | |
| Aplastic anemia | 11 | 100.0 | 0 | 0.0 | |
| Malignancy status | |||||
| Controlled disease | 768 | 93.5 | 53 | 6.5 | <.001 |
| Complete remission | 505 | 96.4 | 19 | 3.6 | |
| Partial remission | 263 | 88.6 | 34 | 11.4 | |
| Stable disease | 294 | 91.3 | 28 | 8.7 | |
| Active disease | 307 | 96.3 | 58 | 3.7 | |
| Onset | 165 | 89.2 | 20 | 10.8 | |
| Refractory/resistant | 142 | 78.9 | 38 | 21.1 | |
| Unknown | 36 | 90.0 | 4 | 10.0 | |
| Last malignancy treatment before COVID-19 | |||||
| Allo-HSCT | 72 | 94.8 | 4 | 5.2 | ns |
| Auto-HSCT | 16 | 100.0 | 0 | 0.0 | |
| CAR-T | 6 | 75.0 | 2 | 25.0 | |
| Conventional chemotherapy | 215 | 90.6 | 91.9 | 8.1 | |
| Demethylating agents | 73 | 90.5 | 7 | 9.5 | |
| Immuno-chemotherapy | 512 | 91.2 | 50 | 8.8 | |
| Immunotherapy | 78 | 87.6 | 11 | 12.3 | |
| Targeted therapy | 279 | 89.8 | 32 | 10.2 | |
| Supportive measures | 28 | 77.8 | 8 | 22.2 | |
| No treatment | 126 | 92.6 | 10 | 7.4 | |
| SARS-CoV-2 vaccination before COVID-19∗ | |||||
| 1 dose | 115 | 89.1 | 14 | 10.9 | ns |
| 2 doses | 689 | 89.5 | 81 | 10.5 | |
| 3 doses | 591 | 91.9 | 48 | 8.1 | |
| 4 doses | 10 | 100.0 | 0 | 0.0 | |
| Type of SARS-CoV-2 vaccine | |||||
| mRNA | 1250 | 90.8 | 127 | 9.2 | ns |
| BioNTech/Pfizer | 1011 | 90.2 | 110 | 9.8 | |
| Moderna COVE | 239 | 93.4 | 17 | 6.6 | |
| Vector-based | 123 | 92.5 | 10 | 7.5 | |
| AstraZeneca Oxford | 91 | 91.9 | 8 | 8.1 | |
| Sputnik | 13 | 100.0 | 0 | 0.0 | |
| J&J (Janssen) | 19 | 90.5 | 2 | 9.5 | |
| Inactivated | 32 | 84.3 | 6 | 15.7 | |
| CoronaVac/Sinovac | 18 | 85.7 | 3 | 14.3 | |
| Sinopharm | 14 | 82.4 | 3 | 17.6 | |
| Spike protein dosage after vaccination† | |||||
| No response | 118 | 87.4 | 17 | 12.6 | ns |
| Weak response | 31 | 91.2 | 3 | 8.8 | |
| Optimal response | 71 | 94.7 | 4 | 5.3 | |
| Not tested | 1185 | 90.9 | 119 | 9.1 | |
| COVID-19 variant | |||||
| Wild type | 36 | 90.0 | 4 | 10.0 | ns |
| Alpha | 30 | 88.2 | 4 | 11.8 | |
| Beta | 1 | 100.0 | 0 | 0.0 | |
| Delta | 141 | 87.6 | 20 | 12.4 | |
| Omicron | 476 | 92.1 | 41 | 7.9 | |
| Not tested | 721 | 90.7 | 74 | 9.3 | |
| COVID treatment | |||||
| No specific treatment reported | 618 | 96.3 | 24 | 3.7 | <.001 |
| Antivirals + monoclonal antibodies | 98 | 90.7 | 10 | 9.3 | |
| Antivirals | 186 | 85.3 | 32 | 14.7 | |
| Corticosteroids | 185 | 75.2 | 61 | 24.8 | |
| Monoclonal antibodies | 302 | 97.1 | 9 | 2.9 | |
| Plasma | 16 | 69.6 | 7 | 30.4 | |
| COVID-19 infection | |||||
| Asymptomatic | 270 | 95.5 | 13 | 4.5 | .002 |
| Mild infection | 581 | 96.1 | 23 | 3.9 | |
| Severe infection | 456 | 89.6 | 53 | 10.4 | |
| Critical infection | 98 | 64.5 | 54 | 35.5 | |
| COVID-19 symptoms | |||||
| Pulmonary | 473 | 89.6 | 55 | 10.4 | .002 |
| Pulmonary + extrapulmonary | 349 | 87.3 | 51 | 12.8 | |
| Extrapulmonary | 297 | 94.6 | 17 | 5.4 | |
| Asymptomatic | 286 | 93.5 | 20 | 6.5 | |
CAR-T, chimeric antigen receptor T cells; HSCT, hematopoietic stem cell transplantation; ns, not statistically significant.
1 to 2 doses vs 3 to 4 doses; P = .040.
Referring to the World Health Organization international standards, binding antibody units per milliliter (https://www.who.int/news-room/feature-stories/detail/standardization-of-vaccines-for-coronavirusdisease-covid-19).
Figure 2.
Survival probability by most prevalent underlying condition.
Table 3.
Univariable and multivariable analysis of factors influencing 30-day mortality
| Univariable |
Multivariable |
|||||||
|---|---|---|---|---|---|---|---|---|
| P value | HR | 95% CI |
P value | HR | 95% CI |
|||
| Lower | Upper | Lower | Upper | |||||
| Sex | ||||||||
| Female | — | — | — | — | ||||
| Male | .148 | 0.785 | 0.566 | 1.090 | ||||
| Age | <.001 | 1.059 | 1.044 | 1.075 | <.001 | 1.042 | 1.024 | 1.061 |
| Malignancy status at COVID-19 diagnosis | ||||||||
| Controlled disease | — | — | — | — | — | — | — | — |
| Stable disease | .183 | 1.364 | 0.863 | 2.157 | .767 | 1.081 | 0.647 | 1.806 |
| Active disease | <.001 | 2.494 | 1.718 | 3.619 | .001 | 1.981 | 1.305 | 3.008 |
| Baseline malignancy | ||||||||
| Aplastic anemia | — | — | — | — | ||||
| Lymphoid malignancies | .875 | 3032.714 | 0.000 | — | ||||
| Myeloid malignancies | .876 | 2974.523 | 0.000 | — | ||||
| Comorbidities | ||||||||
| 0-1 comorbidities | — | — | — | — | — | — | — | — |
| ≥2 comorbidities | <.001 | 2.802 | 2.019 | 3.889 | .027 | 1.503 | 1.050 | 2.229 |
| Type of last vaccination | ||||||||
| mRNA | — | — | — | — | ||||
| Vector-based | .359 | 0.740 | 0.389 | 1.409 | ||||
| Inactivated | .122 | 1.907 | 0.841 | 4.326 | ||||
| SARS-CoV-2 | ||||||||
| Omicron | — | — | — | — | ||||
| Alpha | .800 | 1.142 | 0.409 | 3.190 | ||||
| Beta | .960 | 0.000 | 0.000 | — | ||||
| Delta | .210 | 1.408 | 0.825 | 2.403 | ||||
| Wild type | .758 | 1.175 | 0.421 | 3.281 | ||||
| Not tested | .399 | 1.179 | 0.805 | 1.726 | ||||
| Vaccine doses before COVID-19 | ||||||||
| 1 dose | — | — | — | — | ||||
| 2 doses | .870 | 1.049 | 0.595 | 1.849 | ||||
| ≥3 doses | .637 | 0.866 | 0.478 | 1.572 | ||||
| Serological response before COVID-19 | ||||||||
| No response | — | — | — | — | ||||
| Weak response | .632 | 0.740 | 0.217 | 2.529 | ||||
| Optimal response | .124 | 0.425 | 0.143 | 1.264 | ||||
| COVID-19 treatment | ||||||||
| Corticosteroids | — | — | — | — | — | — | — | — |
| Antivirals + monoclonal antibodies | .001 | 0.333 | 0.171 | 0.651 | .010 | 0.407 | 0.206 | 0.803 |
| Antivirals | .010 | 0.570 | 0.372 | 0.874 | .099 | 0.680 | 0.431 | 1.075 |
| Monoclonal antibodies | <.001 | 0.123 | 0.061 | 0.247 | <.001 | 0.155 | 0.077 | 0.313 |
| Plasma | .852 | 1.077 | 0.493 | 2.355 | .243 | 1.605 | 0.726 | 3.549 |
CI, confidence interval; HR, Hazard ratio.
Figure 3.
Survival probability of patients by COVID-19 treatment.
Figure 4.
Survival probability by COVID-19 treatment and COVID-19 severity. (A) Patients who were asymptomatic; (B) patients with mild disease; (C) patients with severe disease; and (D) patients who were critically ill.
Figure 5.
Survival probability by SARS-CoV-2 variant.
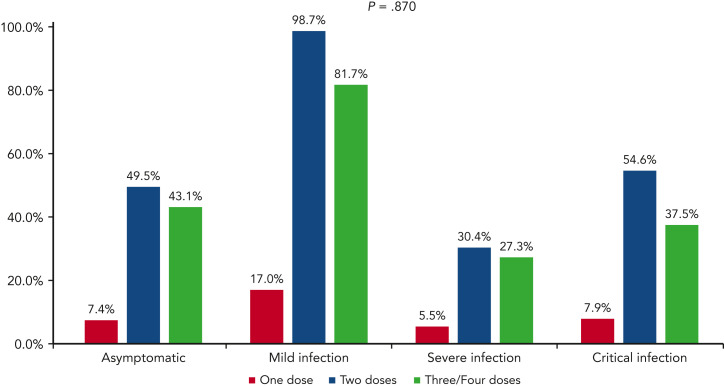
In the multivariable model, older age, active disease, and 2 to 3 comorbidities were the factors significantly correlated with a higher mortality, whereas receiving anti-SARS-CoV-2 treatment with monoclonal antibodies alone or combined with antivirals was independently associated with a lower mortality (HR, 0.155; 95% CI, 0.077-0.313; P < .001 and HR, 0.407; 95% CI, 0.206-0.803; P = .010, respectively) (Table 3). Survival and severity according to vaccine doses administration and postvaccine antispike IgG levels are shown in Figures 6 and 1, respectively.
Figure 6.
Patient distribution by number of SARS-CoV-2 vaccination doses administered before COVID-19 diagnosis, and COVID-19 severity.
Discussion
In the prevaccination era, several studies reported a high COVID-19 mortality in HM.1, 2, 3, 4 From December 2020, anti-SARS-CoV-2 vaccines have been administered in patients with cancer, including those with HM.7 , 8 Most published studies on HM confirmed the efficacy and safety of vaccines, particularly those using mRNA, however, most showing less efficacy in patients with lymphoid malignancies treated with immunosuppressive drugs.17, 18, 19, 20, 21, 22
This study was performed in a large cohort of vaccinated patients with HM to evaluate epidemiology, risk factors for adverse clinical outcome, and treatments of breakthrough COVID-19. We found a predominance of lymphoid malignancies, higher than observed in our previous survey during the prevaccine era; this difference might be explained by the lower efficacy of vaccines in this patient population, as further suggested by the high rate of serological nonresponders among patients with lymphoid malignancies when evaluating antispike IgG levels. These data are consistent with data in a recent report describing COVID-19 breakthrough infections in a large cohort of patients with HM, mostly consisting of patients with lymphoid malignancies.13 Advanced age, presence of comorbidities, and active HM were confirmed in this study as factors that negatively influenced clinical outcome and survival; these were the same risk factors that had previously been reported in the prevaccination era.1, 2, 3, 4 Interestingly, in this study, the underlying malignancy did not have a significant effect on survival, which was different from our previous experience in nonvaccinated patients, where AML and myelodysplastic syndrome were associated with higher mortality risk.4 A potential explanation for this difference might be the better efficacy of anti-SARS-CoV-2 vaccines in myeloid malignancies23, 24, 25 than in lymphoid malignancies;17, 18, 19, 20, 21, 22 however, we may hypothesize new specific anti-SARS-CoV-2 drugs and better COVID-19 management to be particularly important for patients with AML at risk of increased mortality if urgent chemotherapy is delayed. Similarly, as reported by other studies,13 we did not find any significant difference in terms of mortality among different treatments received for HM. As expected, severe and critical COVID-19 had a worse clinical outcome than mild cases, showing a strong correlation with an increased mortality rate both in univariable and multivariable analysis. Given the vaccine protection, the occurrence of respiratory symptoms, hospitalization rate, and severe or critical clinical presentations were significantly lower than in the prevaccination era but still substantially higher compared with that of the overall population.26, 27, 28, 29 However, it is worth underlining that ∼20% of patients were asymptomatic and SARS-CoV-2 infection was detected in screening programs. Interestingly, this percentage is analogous to that reported in our previously published study referring to the prevaccination era.4 Unfortunately, it is not possible to estimate the true incidence of breakthrough infections nor the true number of patients who were asymptomatic with our data as only patients with COVID-19 were included in the registry; this is a potential selection bias, hypothetically hampering the reliability of our results. To the best of our knowledge, only few studies evaluated the incidence and cumulative COVID-19 risk among vaccinated patients with cancer, showing an increased risk in patients with HM compared with the overall population.30, 31, 32 In particular, Lee and coworkers32 recently published a comprehensive population-based test-negative case-control study in the United Kingdom, evaluating COVID-19 breakthrough infections among a large number of vaccinated patients with cancer and healthy control participants. The authors showed that vaccine efficacy at 3 to 6 months after the second dose was lower in the cancer cohort than in the control population and among patients with cancer, was lower in patients with HM, especially those affected by leukemia and lymphoma. Very recently, an Italian study evaluated the immunogenicity and clinical efficacy of anti-SARS-CoV-2 vaccine in 365 patients with HM. The authors showed an overall incidence of breakthrough infections of 2.98 per 10 000 person-days, significantly lower in patients who were seropositive after vaccination, whereas a clear correlation between T-cell immunity response and risk of postvaccine infection has not been found.33
In this study, we reported an overall 30-day-mortality rate of 9.2%, mainly driven by COVID-19 infection as a direct or contributing factor, which is significantly lower than in the prevaccination era.1, 2, 3, 4 Moreover, the 30-day mortality rate in patients who were symptomatic was 10.3%. The success of vaccination strategies is likely a major factor in the reported improvement but not the only factor. Previous reports suggest that COVID-19 management (eg, steroids, etc) have also affected outcomes, and newer variants may be less severe. Data reported in our study are comparable with other recently published reports that showed a significant mortality rate of COVID-19 breakthrough infections among patients with cancer.11 , 12 or more specifically, among those affected by HM.13
In our study, we collected data on viral genotyping in approximately half of patients, among which the most prevalent variant was Omicron, accounting for more than two-thirds of patients. These data are not surprising if we consider the large number of patients registered between late 2021 and early 2022, months in which the Omicron variant was rapidly spreading throughout Europe.34 Interestingly, we did not find any significant difference in terms of severity of clinical presentation and mortality rate between Omicron and other variants, similar to other small, recently published reports on HM35 , 36 but different to reports on immunocompetent patients in which Omicron presents with better outcome than other variants.34 , 37
Most of the patients enrolled in our study received 2 or 3 vaccine doses; comparing clinical presentation and outcomes, we did not find consistent data supporting a better clinical outcome for patients who had received a higher number of vaccine doses, although a slight difference in the proportion of deaths was observed comparing those who received 1 to 2 vs 3 to 4 doses. However, in multivariable analysis, the number of doses did not significantly effect on the overall 30-day mortality. Several studies highlighted the role of a third vaccine dose as capable of restoring the immune response in serologically less responsive patients with HM.38 , 39 However, there are insufficient data to consider patients with low antispike antibody titers at high risk of worse outcomes. Indeed, in our study we did not find any differences in terms of outcomes stratifying patients according to serological response after 2 to 4 weeks from the last vaccine dose. By using the World Health Organization international standards (binding antibody units per milliliter), we did not find a significantly better survival for patients with optimal response, compared with those with weak or no response, although these data were only available in a small percentage of patients (16%). This lack of direct correlation between serological response and survival might be, at least in part, explained by the putative role of anti-SARS-CoV-2–induced cellular immunity, as suggested by several studies,23 , 24 , 40 because the presence of memory T cells might control the infection and prevent severe COVID-19, even if high titers of long-lasting neutralizing antibodies are not elicited.41 However, because a recently published study did not find a clear correlation between postvaccine T-cell immunity and vaccine clinical efficacy,33 further studies are warranted to better understand this aspect. Another possible explanation is related to the role of the specific anti-SARS-CoV-2 treatments (ie, monoclonal antibodies and antivirals) that could have partially balanced the lack of protection of serological nonresponders. Indeed, from our survey, monoclonal antibodies with or without antivirals showed a high clinical activity irrespective of COVID-19 severity, showing the best efficacy when administered as single agents in patients with asymptomatic, mild, or severe disease, and when administered in combination with antivirals in patients with critical disease. The role of monoclonal antibodies in mitigating the negative effect of weak vaccine responses is supported by a recent randomized trial evaluating their role in immunocompetent people without serological response.42 Moreover, our multivariable model confirmed the positive effect on 30-day mortality risk for patients who had received monoclonal antibodies alone or combined with antivirals. We are aware that this study has limitations owing to the retrospective observational design and the possible selection bias owing to the large number of participating institutions. Moreover, viral genotyping and serological data were not available for all enrolled patients, and we did not know whether COVID-19 was first diagnosed in hospital or in the community, potential key information for discriminating patient risk and infection natural history. Further prospective studies that better evaluate the role of vaccine response in patients with HM are needed.
In conclusion, our survey has shown that vaccination and novel COVID-19 treatments have brought significant improvements in terms of mortality in patients with HM. To further improve the prognosis of these patients, the role of additional booster vaccine doses and the role of prophylactic monoclonal antibodies in patients with an ineffective response to vaccination should be investigated.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the EPICOVIDEHA Survey collaborators appears in “Appendix.”
Acknowledgment
The authors thank Janina Leckler and all contributors for their utmost contributions and support to the project during a pandemic situation.
EPICOVIDEHA has received funds from Optics COMMIT (COVID-19 Unmet Medical Needs and Associated Research Extension) COVID-19 RFP program by GILEAD Science, United States (Project 2020-8223).
Authorship
Contribution: L.P. served as the principal investigator; J.S.-G. and F.M. served as project manager and research assistant, respectively. L.P., J.S.-G., and F.M. contributed to study design, study supervision, and data interpretation and wrote the paper; A.B., P.C., M.H., P.K., A.P., F.P., O.A.C., and L.P. conceived the registry idea. L.P., J.S.-G., and F.M. did the statistical plan, analysis, and interpreted the data; all the authors recruited participants and collected and interpreted data; all authors contributed to manuscript writing and review of the manuscript; and all authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
∗L.P., J.S.-G., and F.M. are joint first authors.
Data are available on request from the corresponding author, Livio Pagano (livio.pagano@unicatt.it).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Contributor Information
EPICOVIDEHA Survey members:
Laura Serrano, José-María Ribera-Santa Susana, Joseph Meletiadis, Panagiotis Tsirigotis, Nicola Coppola, Malgorzata Mikulska, Nurettin Erben, Caroline Besson, Maria Merelli, Tomás-José González-López, Jorge Loureiro-Amigo, Carolina García-Vidal, Elizabeth de Kort, Annarosa Cuccaro, Sofia Zompi, Florian Reizine, Olimpia Finizio, Rémy Duléry, Maria Calbacho, Ghaith Abu-Zeinah, Sandra Malak, Przemyslaw Zdziarski, Gina Varrichio, Athanasios Tragiannidis, Gaëtan Plantefeve, Rafael Duarte, François Danion, Maria Chiara Tisi, Ioanna Sakellari, Meinholf Karthaus, Ana Groh, Monica Fung, Ziad Emarah, Omar-Francisco Coronel-Ayala, Louis Yi Ann Chai, Mathias Brehon, Valentina Bonuomo, Dominik Wolf, Jana Wittig, Maria Vehreschild, Mario Virgilio Papa, Julia Neuhann, María-Josefa Jiménez-Lorenzo, Jan Grothe, Eleni Gavriilaki, Ramón García-Sanz, Nicole García-Poutón, Shaimaa Saber El-Ashwah, Matthias Eggerer, Raul Cordoba, Gökçe Melis Çolak, and Elena Arellano
Appendix
The EPICOVIDEHA Survey collaborators that contributed to this work are Laura Serrano, José-María Ribera-Santa Susana, Joseph Meletiadis, Panagiotis Tsirigotis, Nicola Coppola, Malgorzata Mikulska, Nurettin Erben, Caroline Besson, Maria Merelli, Tomás-José González-López, Jorge Loureiro-Amigo, Carolina García-Vidal, Elizabeth de Kort, Annarosa Cuccaro, Sofia Zompi, Florian Reizine, Olimpia Finizio, Rémy Duléry, Maria Calbacho, Ghaith Abu-Zeinah, Sandra Malak, Przemyslaw Zdziarski, Gina Varrichio, Athanasios Tragiannidis, Gaëtan Plantefeve, Rafael Duarte, François Danion, Maria Chiara Tisi, Ioanna Sakellari, Meinholf Karthaus, Ana Groh, Monica Fung, Ziad Emarah, Omar-Francisco Coronel-Ayala, Louis Yi Ann Chai, Mathias Brehon, Valentina Bonuomo, Dominik Wolf, Jana Wittig, Maria Vehreschild, Mario Virgilio Papa, Julia Neuhann, María-Josefa Jiménez-Lorenzo, Jan Grothe, Eleni Gavriilaki, Ramón García-Sanz, Nicole García-Poutón, Shaimaa Saber El-Ashwah, Matthias Eggerer, Raul Cordoba, Gökçe Melis Çolak, and Elena Arellano.
Supplementary Material
References
- 1.Wood WA, Neuberg DS, Thompson JC, et al. Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH Research Collaborative Data Hub. Blood Adv. 2020;4(23):5966–5975. doi: 10.1182/bloodadvances.2020003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with hematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagano L, Salmanton-García J, Marchesi F, et al. COVID-19 infection in adult patients with hematologic malignancies: a European Hematology Association Survey (EPICOVIDEHA) J Hematol Oncol. 2021;14(1):168. doi: 10.1186/s13045-021-01177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Hematology Association (EHA) Expert opinions for COVID-19 vaccination in patients with hematologic cancer. https://ehaweb.org/covid-19/eha-statement-on-covid-19-vaccines/recommendations-for-covid-19-vaccination-in-patients-with-hematologic-cancer/
- 8.Committee NCCNC-VA Preliminary recommendations of the NCCN-COVID-19 Vaccination Advisory Committee. https://www.nccn.org/covid19/pdf/COVID19_Vaccination_Guidance_V1.0.pdf
- 9.Cesaro S, Ljungman P, Mikulska M, et al. Recommendations for the management of COVID-19 in patients with hematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference of Infections in Leukaemia (ECIL-9) Leukemia. 2022;36(6):1467–1480. doi: 10.1038/s41375-022-01578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagano L, Salmanton-García J, Marchesi F, et al. COVID-19 in vaccinated adult patients with hematological malignancies. Preliminary results from EPICOVIDEHA. Blood. 2022;139:1588–1592. doi: 10.1182/blood.2021014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Q, Bates B, Shao YR, et al. Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-world evidence from the National COVID Cohort Collaborative. J Clin Oncol. 2022;40(13):1414–1427. doi: 10.1200/JCO.21.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt AL, Labaki C, Hsu CY, et al. COVID-19 vaccination and breakthrough infections in patients with cancer. Ann Oncol. 2022;33(3):340–346. doi: 10.1016/j.annonc.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Kaelber DC, Xu R, Berger NA. COVID-19 breakthrough infections, hospitalizations and mortality in fully vaccinated patients with hematologic malignancies: a clarion call for maintaining mitigation and ramping-up research. Blood Rev. 2022;54 doi: 10.1016/j.blre.2022.100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmanton-García J, Busca A, Cornely OA, et al. EPICOVIDEHA: a ready to use platform for epidemiological studies in hematological patients with COVID-19. Hemasphere. 2021;5:e612. doi: 10.1097/HS9.0000000000000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.COVID-19 clinical management. Living guidance World Health Organization. 2021. https://apps.who.int/iris/handle/10665/338882
- 16.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 17.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell. 2021;39(10):1297–1299. doi: 10.1016/j.ccell.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghione P, Gu JJ, Attwood K, et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell directed therapies. Blood. 2021;138(9):811–814. doi: 10.1182/blood.2021012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchesi F, Pimpinelli F, Giannarelli D, et al. Impact of anti-CD20 monoclonal antibodies on serologic response to BNT162b2 vaccine in B-cell Non-Hodgkin’s lymphomas. Leukemia. 2022;36(2):588–590. doi: 10.1038/s41375-021-01418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzog Tzarfati K, Gutwein O, Apel A, et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96(10):1195–1203. doi: 10.1002/ajh.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry C, Luttwak E, Balaban R, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053–3061. doi: 10.1182/bloodadvances.2021005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington P, Doores KJ, Radia D, et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces neutralising antibody and polyfunctional T-cell responses in patients with chronic myeloid leukemia. Br J Haematol. 2021;194(6):999–1006. doi: 10.1111/bjh.17568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington P, de Lavallade H, Doores KJ, et al. Single dose of BNT162b2 mRNA vaccine against SARS-CoV-2 induces high frequency of neutralising antibody and polyfunctional T-cell responses in patients with myeloproliferative neoplasms. Leukemia. 2021;35(12):3573–3577. doi: 10.1038/s41375-021-01300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pimpinelli F, Marchesi F, Piaggio G, et al. Lower response to BNT162b2 vaccine in patients with myelofibrosis compared to polycythemia vera and essential thrombocythemia. J Hematol Oncol. 2021;14(1):119. doi: 10.1186/s13045-021-01130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1059–1062. doi: 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Clin Infect Dis. 2022;74(9):1515–1524. doi: 10.1093/cid/ciab687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin JB, Haddix M, Danza P, et al. SARS-CoV-2 infections and hospitalizations among persons aged ≥16 years, by vaccination status - Los Angeles County, California, May 1-July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1170–1176. doi: 10.15585/mmwr.mm7034e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Kaelber DC, Xu R, Berger NA. Breakthrough SARS-CoV-2 infections, hospitalizations, and mortality in vaccinated patients with cancer in the US between December 2020 and November 2021. JAMA Oncol. 2022;8(7):1027–1034. doi: 10.1001/jamaoncol.2022.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittelman M, Magen O, Barda N, et al. Effectiveness of the BNT162b2mRNA vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood. 2022;139(10):1439–1451. doi: 10.1182/blood.2021013768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee LYW, Starkey T, Ionescu MC, et al. Vaccine effectiveness against COVID-19 breakthrough infections in patients with cancer (UKCCEP): a population-based test-negative case-control study. Lancet Oncol. 2022;23(6):748–757. doi: 10.1016/S1470-2045(22)00202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvini M, Damonte C, Mortara L, et al. Immunogenicity and clinical efficacy of anti-SARS-CoV-2 vaccination in patients with hematological malignancies: results of a prospective color study of 365 patients. Am J Hematol. 2022;97(8):E321–E324. doi: 10.1002/ajh.26629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niemann CU, da Cunha-Bang C, Helleberg M, Ostrowski SR, Brieghel C. Patients with CLL have a lower risk of death from COVID-19 in the Omicron era. Blood. 2022;140(5):445–450. doi: 10.1182/blood.2022016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taenaka R, Obara T, Kohno K, Aoki K, Ogawa R. Infections with the SARS-CoV-2 Omicron variant show a similar outcome as infections with the previous variants in patients with hematologic malignancies. Ann Hematol. 2022;101(8):1877–1878. doi: 10.1007/s00277-022-04833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen PA, Olsen RJ, Long SW, et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with Coronavirus disease 2019 caused by the Omicron variant of severe acute respiratory syndrome Coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192(4):642–652. doi: 10.1016/j.ajpath.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Šušol O, Hájková B, Zelená H, Hájek R. Third dose of COVID-19 vaccine restores immune response in patients with hematological malignancies after loss of protective antibody titres. Br J Haematol. 2022;197(3):302–305. doi: 10.1111/bjh.18073. [DOI] [PubMed] [Google Scholar]
- 39.Mair MJ, Berger JM, Mitterer M, et al. Third dose of SARS-CoV-2 vaccination in hemato-oncological patients and health care workers: immune responses and adverse events - a retrospective cohort study. Eur J Cancer. 2022;165:184–194. doi: 10.1016/j.ejca.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marasco V, Carniti C, Guidetti A, et al. T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol. 2022;196(3):548–558. doi: 10.1111/bjh.17877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.RECOVERY collaborative group RC Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399(10325):665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.