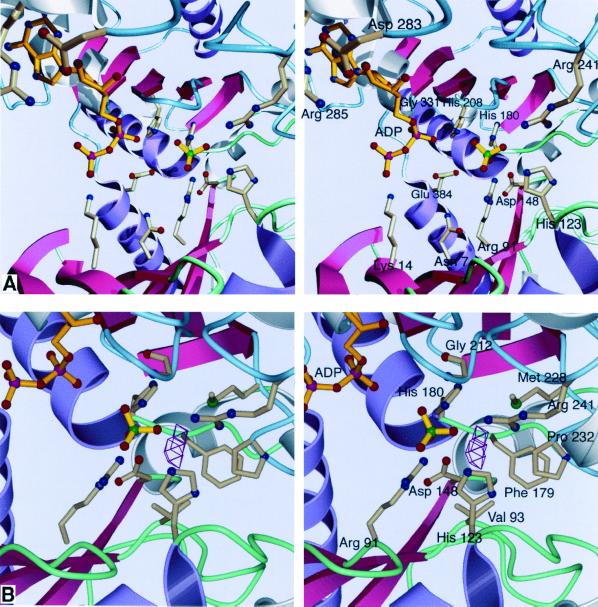
FIG. 4.
Stereoviews of the active site of acetate kinase. Conserved ASKHA secondary structures are pink and purple, and inserted secondary-structural elements are gray. Loops in the N-terminal domain are green, and those in the C-terminal domain are blue. (A) The binding site. It is likely that the sulfate occupies the position where the phosphate of acetyl phosphate would bind. No magnesium ion is apparent in our current structure despite the inclusion of 750 μM MgCl2 in the crystallization conditions. (B) The proposed site of acetate binding. VOIDOO (27) was used to locate solvent-accessible cavities. The cavity shown could easily accommodate the methyl group of acetate or acetyl phosphate, positioning the phosphate roughly where the sulfate is located. As shown, the center of the cavity is 4.2 Å away from the sulfate.