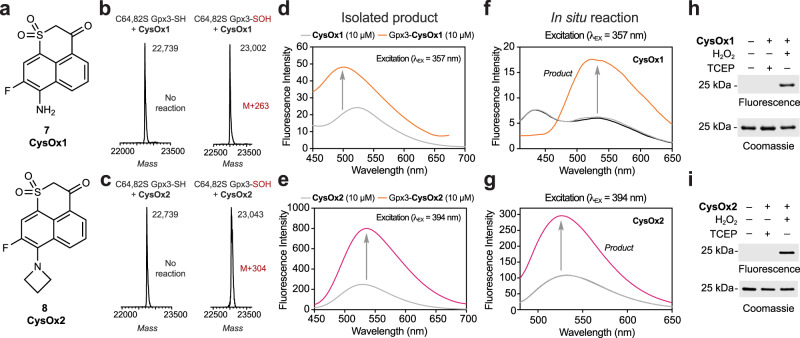
Fig. 3. CysOx1 and CysOx2 are reaction-based fluorogenic probes for sulfenic acid.
a Structures of CysOx1 and CysOx2. b, c Intact MS analysis of the reaction products of reduced or sulfenic acid forms of C64,82 S Gpx3 (10 µM) with CysOx1 or CysOx2 (1 mM) after 1 h in 50 mM HEPES pH 7.4. d, e Emission spectra of CysOx1 or CysOx2 alone (10 µM) compared to purified Gpx3-CysOx1 or Gpx3-CysOx2 (10 µM). Reactions with CysOx1 or CysOx2 were excited at 357 nm and 394 nm, respectively. Spectra were recorded in 50 mM HEPES pH 7.4. f, g Emission spectra of CysOx1 or CysOx2 alone (5 µM) or in combination with H2O2 (15 µM) or Gpx3 (5 µM) or H2O2 (15 µM) and Gpx3 (5 µM). Only the reaction containing all three components gives a fluorogenic product. Reactions with CysOx1 or CysOx2 were excited at 357 and 394 nm, respectively. Spectra were recorded in 50 mM HEPES pH 7.4. Analysis by intact MS indicates that ~75% Gpx3-SOH is labeled by CysOx probes under these conditions. h, i, In-gel fluorescence analysis of reaction products between Gpx3 (10 µM) and CysOx1 or CysOx2 (1 mM) with or without H2O2 (15 µM) or TCEP (1 mM) after 1 h in 50 mM HEPES pH 7.4. N = 2 independent experiments for each probe.