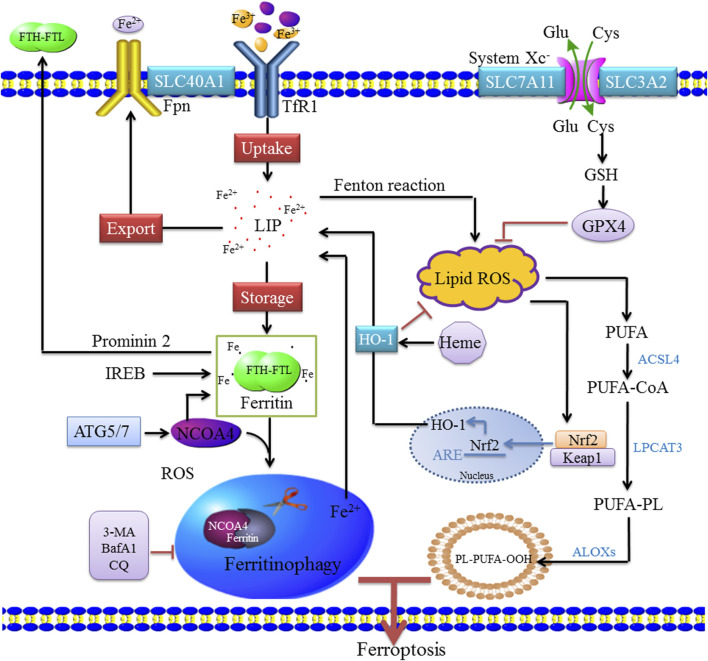
FIGURE 1.
Molecular mechanisms of ferritinophagy. Extracellular Fe3+ relies on Tfr1 for transferring into the cell to form Fe2+, where it binds to form a ferritin storage cage, which is composed of FTL and FTH. Fe3+ is reduced to Fe2+ in the endosome by STEAP3 (iron reductase), which then mediates the release of Fe2+ from the endosome into the cytoplasm via DMT1/SLC11A2 to form LIP. Cytoplasmic Fe2+ can be reduced by ferritin and stored as Fe3+, and Fe2+ can be transported out of the cell by ferroportin (FPN1/SLC40A1). These mechanisms of controlling iron intake, storage, and export maintain the amount of iron in the redox-active LIP. When bioavailable iron levels are low, the body regulates the release of iron and replenishes ferritin through selective autophagy degradation. When autophagy is activated, NCOA4 mediates the binding of ferritin to the lysosome, causing it to degrade and release free iron. NCOA4 promotes its autophagic degradation by binding ferritin, leading to the formation of ferritinophagy, whereas the inhibition of autophagy leads to the accumulation of NCOA4. Ferritinophagy leads to increased intracellular iron levels and ROS accumulation through the Fenton reaction, leading to ferroptosis. ACSL4 is involved in the biosynthesis of PUFAs and helps free PUFAs to be esterified and incorporated into membrane phospholipids. Nrf2 activates HO-1, which leads to an increase in heme degradation. An imbalance among these homeostatic factors could lead to an accumulation of excess intracellular iron, increased ROS production, and induction of cell death.