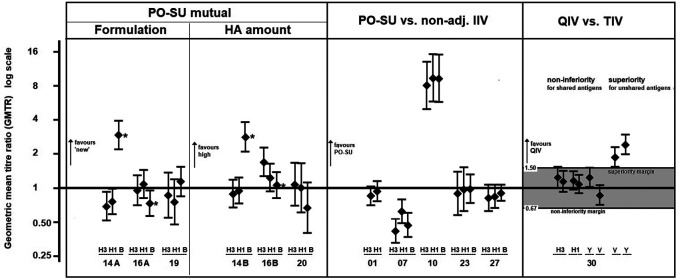
Figure 4. Randomised comparison trials (AZB-SU versus three comparator classes).
Geometric mean titre ratios.
Legend: AZB-SU mutual: T14A: AZB-SU 2008 vs. AZB-SU 1996; T16A: AZB-SU TC vs. AZB-SU 1996; T19: AZB-SU TC vs. PO- SU 2008; T14B: AZB-SU 2008 10 μg HA vs. AZB-SU 1996 5 μg; T16B: AZB-SU TC 10 μg HA vs. AZB-SU 1996; T20: AZB-SU 2008 10 μg HA vs AZB-SU 2008. AZB-SU vs. non-adjuvanted inactivated influenza vaccine (IIV): T01, T07, T10: AZB-SU1 996; T23, T27: AZB-SU 2008. QIV vs. TIV: QIV, quadrivalent influenza vaccine; TIV: trivalent influenza vaccine; Y, B/Yamagata; V: B/Victoria. Non-inferiority is demonstrated if the lower limit of the 95% confidence interval around the GMTR value (QIV versus TIV) is larger than the pre-defined non-inferiority margin of 0.67. Superiority is demonstrated if the lower limit of the 95% CI around the GMTR is larger than the pre-defined superiority margin of 1.5.
* atypical influenza B component in 2008.