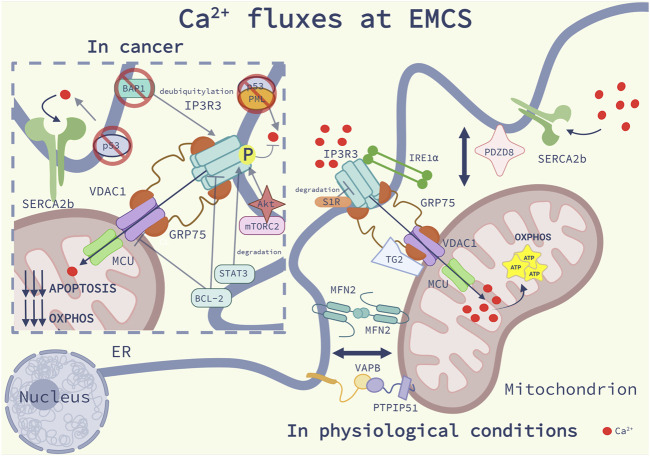
FIGURE 1.
Ca2+ fluxes at EMCS: Schematic representation of the main modulators of Ca2+ homeostasis at ER-mitochondria contact sites (EMCS) in physiological conditions (on the right) and in cancer (on the left), as discussed in the main text. On the right side of the image, are represented the main Ca2+ regulatory systems at EMCS: the inositol 1,4,5-trisphosphate receptors 3 (IP3R3)- glucose-related regulated protein 75 (Grp75)-voltage-dependent anion channel 1 (VDAC1) signaling complex, delivering Ca2+ to the mitochondria, the Ca2+ uniporter (MCU), allowing Ca2+entry into the mitochondrial matrix, and the sarco/endoplasmic reticulum Ca2+ ATPase 2b (SERCA2b), which pumps Ca2+ from the cytosol to the ER. Maintenance of mitochondrial Ca2+ homeostasis through the integrity of the EMCS, promotes mitochondrial bioenergetics. Sigma-1Receptor (S1R) binds to IP3R3 and decreases its degradation; the ER stress sensor Inositol-requiring enzyme 1 (IRE1α) interacts and brings IP3R3 at EMCS. Transglutaminase type 2 (TG2) binds to Grp75 and promotes mitochondrial Ca2+ uptake. The complex formed by the protein tyrosine phosphatase interacting protein 51 (PTPIP51) and the vesicle-associated membrane protein-associated protein B (VAPB), PDZ domain-containing protein 8 (PDZD8) and Mitofusin 2 (MFN2) homodimers favor Ca2+ fluxes by regulating the EMCS proximity. On the left side of the image we describe Ca2+ dysregulation in cancer. The tumor suppressor p53 at EMCS binds to SERCA pump preventing its ROS-mediated inactivation. P53 interacts with promyelocytic leukemia (PML) protein, promoting the dephosphorylation and inactivation of the proto-oncogene serine/threonine kinase Akt, which together with the Rapamycin complex 2 (mTORC2) phosphorylate and inhibit IP3R3. The proto-oncogene B-cell lymphoma 2 (Bcl-2) interacts and inhibits VDAC1 and IP3R3 suppressing Ca2+ fluxes. IP3R3 stability is regulated by the oncogenic signal transducer and activator of transcription 3 (STAT3) which promotes its degradation and the tumor suppressor deubiquitylase BRCA1-associated protein 1 (BAP1), which prevents its ubiquitylation. The mechanisms described here contribute to a reduction of mitochondrial Ca2+ import, and consequently to a lower oxidative phosphorylation (OXPHOS) and reduced apoptosis sensitivity.