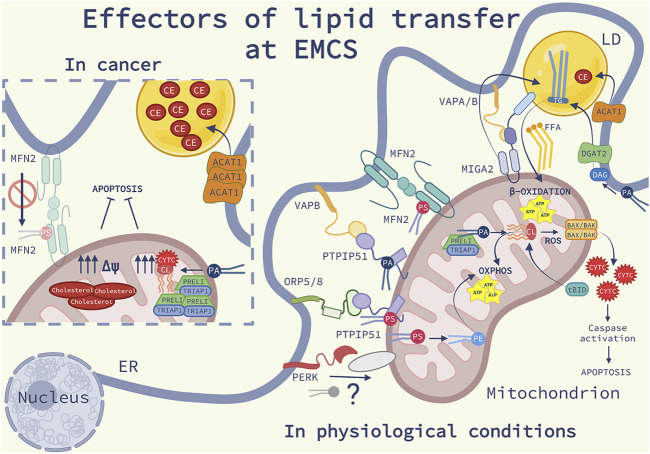
FIGURE 2.
Effectors of lipid transfer at EMCS: Schematic representation of the lipid homeostasis at ER-mitochondria contact sites (EMCS) and lipid droplets (LD), in physiological conditions (on the right) and in cancer (on the left). On the right side of the image, are represented the main lipid transfer proteins (LTP) discussed in the review (see the main text for further explanations); Mitofusin 2 (MFN2) homodimers, bind and transfer phosphatidylserine (PS), the oxysterol-binding protein-related protein (ORP) 5 and 8-protein- tyrosine phosphatase interacting protein 51 (PTPIP51) complex, the vesicle-associated membrane protein-associated protein B (VAPB)- PTPIP51 complex, responsible of the transfer of phosphatidic acid (PA). The Ser/Thr kinase RNA-dependent protein kinase (PKR)-like ER kinase (PERK) is shown to interact with an unknown mitochondrial protein, possibly facilitating phospholipid (PL) transport. In the inner mitochondrial membrane (IMM) PS is converted into PE and PA and transferred into the mitochondrial intermembrane space (IMS) by TP53-regulated inhibitor of apoptosis gene 1 TRIAP1-PRELI complex. PA is converted into cardiolipin (CL), an anionic PL which promotes mitochondrial oxidative phosphorylation (OXPHOS). In response to cell death stimuli, CL interacts with truncated BH3 interacting-domain death agonist (tBID) promoting BAX/BAK oligomerization, release of cytochrome c (CYTC), triggering caspase activation and apoptosis. Mitoguardin 2 (MIGA2)-VAPA/B complex tethers the EMCS to LD and facilitates the production of triglycerides (TG). TG is synthesized into the ER from PA via the diacylglycerol acyltransferase 2 (DGAT2) enriched at EMCS. The enzyme acyl-CoA cholesterol acyltransferase-1 (ACAT1) localized at EMCS synthetizes cholesteryl esters (CE). TG and CE are stored in LD. TG hydrolysis provides free fatty acids (FFA) as fuel for β-oxidation occurring in the mitochondria. On the left panel, some molecular mechanisms involving lipid signaling at EMCS, which are altered in cancer cells, are illustrated: ACAT1 expression is increased, consequently leading to the accumulation of elevated levels of CE into LD; the complex TRIPA1-PRELI is upregulated, PA is converted into CL, impairing the release of CYTC and the sensitivity to apoptosis; cholesterol is highly accumulated into the IMM, affecting the mitochondrial potential (Δψ) and favoring resistance to apoptosis. Deficiency of MFN2 compromises the transferring of PS at EMCS causing ER stress, inflammation, fibrosis and cancer.