Abstract
Background
Pregnant women are recommended to receive coronavirus disease 2019 (COVID-19) vaccines; however, relative effectiveness of vaccination by pregnancy status is unclear.
Methods
We compared the relative effectiveness of messenger RNA (mRNA) COVID-19 vaccines according to whether women received both doses while pregnant (n = 7412), 1 dose while pregnant (n = 3538), both doses while postpartum (n = 1856), or both doses while neither pregnant nor postpartum (n = 6687). We estimated risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection starting 14 days after the second dose using Cox regression, reporting hazard ratios (HRs) and 95% confidence intervals (CIs). Second, we examined relative effectiveness of a third (booster) dose while pregnant compared to outside pregnancy. The major circulating variant during the study period was the Delta variant.
Results
Fifty-four percent of women received 2 doses of the BNT162b2 vaccine, 16% received 2 doses of the mRNA-1273 vaccine, while 30% received 1 dose of both vaccines. Compared to women who received both doses while neither pregnant nor postpartum, the adjusted HR for a positive SARS-CoV-2 polymerase chain reaction test was similar if the woman received both doses while pregnant (1.04 [95% CI, .94–1.17]), 1 dose while pregnant and 1 dose before or after pregnancy (1.03 [95% CI, .93–1.14]), or both doses while postpartum (0.99 [95% CI, .92–1.07]). The findings were similar for BNT162b2 (Pfizer-BioNTech Comirnaty) and mRNA-1273 (Moderna Spikevax), and during Delta- and Omicron-dominant periods. We observed no differences in the relative effectiveness of the booster dose according to pregnancy status.
Conclusions
We observed similar effectiveness of mRNA vaccines against SARS-CoV-2 infection among women regardless of pregnancy status at the time of vaccination.
Keywords: COVID-19, vaccination, pregnancy, postpartum
In this observational population-based study, there was a similar effectiveness of BNT162b2 and mRNA-1273 vaccines against SARS-CoV-2 infection among women regardless of pregnancy status at the time of vaccination.
Coronavirus disease 2019 (COVID-19) vaccines were developed at an unprecedented rate, and randomized controlled trials confirmed high vaccine efficacy against the wild-type strain [1, 2]. Pregnant women were excluded from prelicensure COVID-19 vaccine trials; thus, effectiveness and safety during pregnancy must be evaluated in postlicensure studies [3, 4]. Since pregnant women have a higher risk of severe COVID-19 disease [5, 6], and no evidence of increased adverse outcomes after vaccination [7–9], a general recommendation for COVID-19 vaccination of pregnant women was issued [10, 11].
A meta-analysis of observational studies (2 from Israel and 1 from Qatar) [12–14] that included 19 828 vaccinated and 18 828 unvaccinated pregnant women reported a 90% effectiveness of messenger RNA (mRNA) vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection 1 week after the second dose [9]. There was heterogeneity in the magnitude of the vaccine effectiveness across the individual studies [12–14], which were all conducted in pre-Delta time periods; however, within counties, estimates were comparable to the general adult population during similar time periods [9].
Although studies show similar immunogenicity of mRNA COVID-19 vaccines in pregnant, lactating, and nonpregnant women [15, 16], comparisons of effectiveness among these 3 population groups are lacking. The objective of this study was to compare the relative effectiveness of mRNA COVID-19 vaccines according to pregnancy status at the time of vaccination.
METHODS
This study was approved by the Regional Committee for Medical and Health Research Ethics of South/East Norway (number 141135). The committee provided a waiver of consent for participants due to the registry-based nature of this study.
Study Population
We included 19 679 women in Norway between 15 and 45 years of age who either completed a pregnancy between 2020 and 15 February 2022, or were still pregnant on 15 February 2022, and who had received a second dose of an mRNA COVID-19 vaccine between 1 July and 30 September 2021. We excluded women who had received non-mRNA COVID-19 vaccines (n = 128), as these were not used in Norway's vaccination program, and women who had a positive SARS-CoV-2 test prior to the second vaccine dose (n = 58). We categorized women into 4 exposure groups: (i) received both doses during pregnancy, (ii) received 1 dose while pregnant (and the other dose received before or after pregnancy), (iii) postpartum at the time of vaccination (had been pregnant within 2 months before receiving their first vaccine dose), or (iv) neither pregnant nor postpartum at the time of vaccination (reference group). To ensure the reference group was similar to women vaccinated during pregnancy or in the postpartum period with respect to their demographic characteristics and life stage (ie, family planning), we restricted the reference group to women who had been pregnant during the same calendar period the year before. Data for this study were provided through the emergency preparedness register for COVID-19 (Beredt C19) [17].
Identification of Completed and Ongoing Pregnancies
The birth registry provided data on live births, stillbirths, fetal losses, and induced abortions from 12 gestational weeks onward. We estimated the start of pregnancy by subtracting the estimated gestational age in days from the date of birth. The gestational age was based on ultrasound for 95% of pregnancies and last menstrual period for the remaining 5% of pregnancies. Registrations of miscarriages and induced abortions occurring before 12 gestational weeks were obtained from the patient registry and the general practitioner database [18]. The diagnostic codes used to identify miscarriage and induced abortion are shown in Supplementary Table 1. As these early miscarriages and induced abortions are not registered with a gestational length, we assigned them a gestational duration of 8 weeks, which was based on the mean gestational length for all induced abortions in Norway in the anonymous abortion registry [19] and the gestational age distribution of miscarriages from the literature [20, 21]. The start of these pregnancies ending in a first trimester miscarriage or induced abortion was therefore set to be 8 weeks prior to the event.
We identified ongoing pregnancies using codes for antenatal care visits in the general practitioner database and the patient registry (Supplementary Table 2) [22]. Antenatal codes are not registered with a gestational length. Based on the distribution of the first registration of any pregnancy-related code for completed pregnancies in the birth registry (Supplementary Figure 1), which showed a median of 35 gestational days (5 gestational weeks), we set the start date of ongoing pregnancies to be 5 weeks before the first antenatal consultation.
COVID-19 Vaccination
The Norwegian Immunisation Register (SYSVAK) contains mandatory registration of all COVID-19 vaccinations, with dates of vaccination and vaccine type/product. In Norway, the 2 mRNA vaccines, BNT162b2 (Pfizer-BioNTech Comirnaty) and mRNA-1273 (Moderna Spikevax), were part of the national vaccination program throughout the study period, whereas ASD1222 (AstraZeneca) was excluded from the program on 12 May 2021. General recommendations for vaccination of pregnant women in the second or third trimester were issued in August 2021 in Norway [23]. Prior to this, COVID-19 vaccination of pregnant individuals was only recommended if they were otherwise eligible due to being at high risk of severe COVID-19 or at high risk of acquiring COVID-19 (eg, healthcare providers). Vaccination during the first trimester was not recommended in Norway until mid-January 2022. We categorized women according to whether they received both first and second doses while pregnant, only 1 dose during pregnancy (and the other dose either before or after pregnancy), both doses while postpartum (first dose given during the first 60 days after the end of a pregnancy), or both doses while not pregnant nor postpartum.
SARS-CoV-2 Infection
We obtained information on positive polymerase chain reaction (PCR) tests for SARS-CoV-2 from the Norwegian Surveillance System for Communicable Diseases. This registry includes mandatory reporting for selected infectious diseases, including information on the date of testing and test results for all positive PCR tests for SARS-CoV-2. The number of positive cases has been reported weekly by the Norwegian Institute of Public Health throughout the pandemic [24]. We did not have information on positive antigen tests. There was a general recommendation for everyone with a positive antigen test for SARS-CoV-2 to get a confirmatory PCR test up until 15 February 2022 [25]. After this time, individuals who had received 3 doses of a COVID-19 vaccine, or who had received 2 vaccine doses and experienced an infection with COVID-19, were no longer recommended to do a confirmatory PCR test.
Statistical Analysis
We used Cox proportional hazards regression to compare the incidence of SARS-CoV-2 infection after a second dose between women vaccinated while neither pregnant nor postpartum (reference), women who received both doses during pregnancy, 1 dose during pregnancy, and women who received both doses during the postpartum period. The start of follow-up was 14 days after the second dose of an mRNA COVID-19 vaccine; the time axis for the analysis therefore reflects time in days since the second dose. End of follow-up was the first date of a registered positive test for SARS-CoV-2, death, emigration, or 15 February 2022 for those who were alive and still residing in Norway. The date 15 February was used as the end of follow-up because this was when new guidelines were issued that no longer advised confirmatory PCR testing for those with a positive antigen test. We adjusted for women's age at start of follow-up, education, income, marital status, parity, various underlying chronic medical conditions (diabetes, chronic lung diseases, cerebrovascular disease, other chronic cardiovascular diseases, and reduced immune function due to medication use), and the number of days between the first and second doses. In addition, we adjusted for pregnancy status and booster dose (third dose of an mRNA vaccine) as time-varying covariates. We also conducted stratified analyses according to whether the women had received a homologous primary series of BNT162b2 or mRNA-1273 (those who received a heterologous vaccine series were excluded in this sensitivity analysis; n = 1742). To further examine whether there was any difference according to the circulating SARS-CoV-2 variant, we conducted stratified analyses according to the Delta-dominant period (up until 31 December 2021) and Omicron-dominant period (from 1 January 2022 onward) [26]. These periods were defined based on the major circulating variants nationally. Unfortunately, only a small number of positive PCR tests were genotyped to confirm the strain. This was usually done during a period around the time when a new variant was thought to have been discovered, to identify when a new variant started to circulate nationally. No violations of the proportional hazards assumption were identified based on inspections of the Schoenfeld residuals.
We also compared the relative effectiveness of a booster dose of 1 of the mRNA vaccines (dose 3). This analysis was restricted to women who received the booster from 1 January 2022 onward, because this is when booster doses became available for the general population and not just restricted to elderly or high-risk groups. We compared the risk of a positive PCR test for SARS-CoV-2 according to whether the woman was pregnant, postpartum, or neither when she received the booster. The start of follow-up for this analysis was 14 days after the booster dose was received and follow-up ended on the date of infection, emigration, death, or 15 February 2022. We adjusted for the same characteristics as included in the previous analysis, in addition to number of days between doses 2 and 3.
All analyses were conducted in Stata version 16.0 version (StataCorp, College Station, Texas).
RESULTS
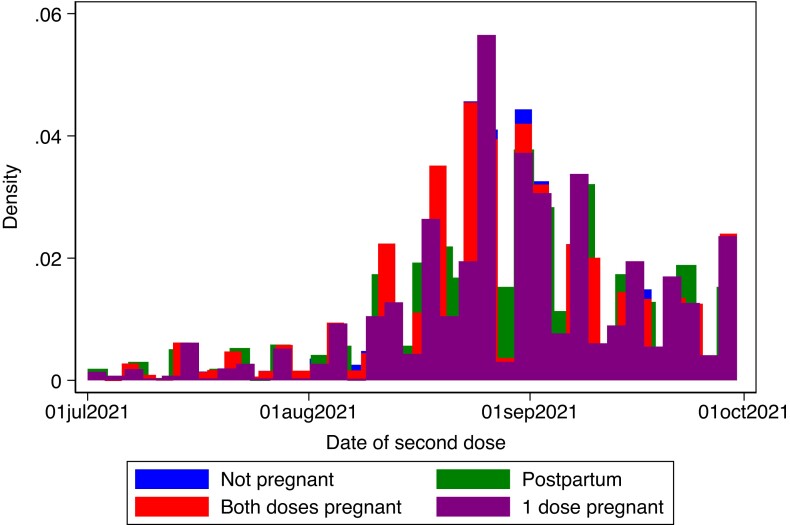
We identified 7412 women who received both dose 1 and dose 2 of an mRNA vaccine during pregnancy, 3538 women who received 1 dose during pregnancy (with the other dose before or after pregnancy), 1856 who received both doses while postpartum, and 6687 women who received both doses while neither pregnant nor postpartum. Fifty-four percent of women received 2 doses of the BNT162b2 vaccine, 16% received 2 doses of the mRNA-1273 vaccine, and 30% received 1 dose of both vaccines. Women who received both doses during pregnancy were slightly younger, more likely to be born in Scandinavia, more likely to have attained higher education, and less likely to be nulliparous compared to women vaccinated while neither pregnant nor postpartum (Table 1). Women vaccinated during the postpartum period, and women who had 1 vaccine dose during and another outside of pregnancy, were similar to women who received both doses while not pregnant or postpartum (Table 1). The calendar timing of dose 2 according to pregnancy status indicates a relatively balanced distribution among the groups (Figure 1).
Table 1.
Background Characteristics According to Pregnancy Status at Time of Vaccination
| Characteristic | Dose 1 and 2 Given While Not Pregnant or Postpartum (n = 6687) | Dose 1 and 2 Given During the Postpartum Period (n = 1856) |
Dose 1 and Dose 2 Given During Pregnancy (n = 7412) |
1 Dose During Pregnancy and 1 Dose Before/After Pregnancy (n = 3538) |
|---|---|---|---|---|
| Age at start of follow-up, y, mean (SD) | 31.5 (6.4) | 31.4 (6.1) | 30.7 (6.0) | 30.9 (5.5) |
| Days between dose 1 and 2, median (IQR) | 42 (34–55) | 42 (34–55) | 42 (34–55) | 49 (41–63) |
| Country of birth, No. (%) | ||||
| ȃScandinavia | 5082 (76.0) | 1452 (78.2) | 5882 (79.4) | 2781 (78.6) |
| ȃOther European countries | 494 (7.4) | 130 (7.0) | 494 (6.7) | 270 (7.6) |
| ȃMiddle East/Africa | 427 (6.4) | 104 (5.6) | 410 (5.5) | 187 (5.3) |
| ȃOther/unknown | 684 (10.2) | 170 (9.2) | 626 (8.5) | 300 (8.5) |
| Marital status, No. (%) | ||||
| ȃMarried/registered partner | 4226 (63.2) | 1173 (63.2) | 4787 (64.6) | 2154 (60.9) |
| ȃUnmarried | 1995 (29.8) | 560 (30.2) | 2201 (29.7) | 1219 (34.5) |
| ȃDivorced/separated | 466 (7.0) | 123 (6.6) | 424 (5.7) | 165 (4.7) |
| Educational level, No. (%) | ||||
| ȃElementary school | 1672 (25.0) | 414 (22.3) | 1725 (23.3) | 582 (16.5) |
| ȃHigh school | 1472 (22.0) | 409 (22.0) | 1548 (20.9) | 723 (20.4) |
| ȃVocational | 133 (2.0) | 26 (1.4) | 105 (1.4) | 49 (1.4) |
| ȃUp to 4 y of higher education | 1933 (28.9) | 558 (30.1) | 2313 (31.2) | 1239 (35.0) |
| ȃ>4 y of higher education | 976 (14.6) | 310 (16.7) | 1239 (16.7) | 702 (19.8) |
| ȃUnknown | 501 (7.5) | 139 (7.5) | 482 (6.5) | 243 (6.9) |
| Household income (NOK), No. (%) | ||||
| ȃ1st tertile (≤500 730) | 2328 (34.8) | 625 (33.7) | 2532 (34.2) | 1144 (32.3) |
| ȃ2nd tertile (500 731–846 668) | 2318 (34.7) | 655 (35.3) | 2712 (36.6) | 1312 (37.1) |
| ȃ3rd tertile (>846 668) | 1848 (27.6) | 495 (26.7) | 1950 (26.3) | 962 (27.2) |
| ȃUnknown | 193 (2.9) | 81 (4.4) | 218 (2.9) | 120 (3.4) |
| Parity | ||||
| ȃ0 | 3049 (45.6) | 835 (45.0) | 3225 (43.5) | 1358 (38.4) |
| ȃ1 | 1470 (22.0) | 469 (25.6) | 1974 (26.6) | 1121 (31.7) |
| ȃ2 | 1427 (21.3) | 386 (20.8) | 1509 (20.4) | 759 (21.5) |
| ȃ≥3 | 741 (11.1) | 166 (8.9) | 704 (9.5) | 300 (8.5) |
| Chronic conditions, No. (%) | ||||
| ȃDiabetes | 52 (0.8) | 12 (0.7) | 68 (0.9) | 30 (0.9) |
| ȃCerebrovascular disease | 9 (0.1) | <5 (0.2) | 6 (0.1) | 5 (0.1) |
| ȃOther chronic cardiovascular disorders | 46 (0.7) | 15 (0.8) | 46 (0.6) | 21 (0.6) |
| ȃReduced immune function due to medications | 79 (1.2) | 18 (1.0) | 86 (1.2) | 50 (1.4) |
| ȃChronic lung disease | 237 (3.5) | 63 (3.4) | 248 (3.4) | 124 (3.5) |
| Healthcare worker | 658 (9.8) | 187 (10.1) | 763 (10.3) | 487 (13.8) |
Abbreviations: IQR, interquartile range; NOK, Norwegian kroner; SD, standard deviation.
Figure 1.
Calendar date of administration of the second messenger RNA coronavirus disease 2019 vaccine according to pregnancy status at the time of vaccination.
Relative Vaccine Effectiveness After the Second Dose of an mRNA Vaccine According to Pregnancy Status
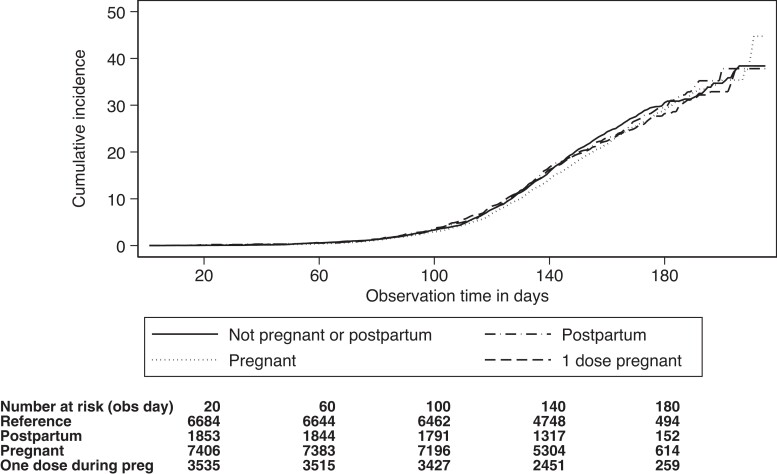
The incidence of SARS-CoV-2 infection per 10 000 follow-up days was 14 among women who received both doses of an mRNA vaccine during pregnancy, 15 among women who received 1 dose during pregnancy, 14 among women who received both doses postpartum, and 15 among women who received both doses while not pregnant or postpartum. Figure 2 shows the cumulative incidence of SARS-CoV-2 infection according to whether the woman was vaccinated while pregnant, postpartum, or neither. In adjusted models, we observed no difference in the risk of SARS-CoV-2 if the woman received both doses while pregnant (adjusted hazard ratio [HR], 0.99 [95% confidence interval {CI}, .92–1.07), 1 dose while pregnant (adjusted HR, 1.03 [95% CI, .93–1.14]), or both doses during the postpartum period (adjusted HR, 1.04 [95% CI, .94–1.17]), as compared to women who were neither pregnant nor postpartum (Table 2). These estimates were similar for the 2 different mRNA vaccines (Table 2). We also did not observe any notable differences during the Delta- and Omicron-dominant periods (Table 3).
Figure 2.
Cumulative incidence of severe acute respiratory syndrome coronavirus 2 infection ≥14 days after the second dose of a messenger RNA (mRNA) coronavirus disease 2019 (COVID-19) vaccine according to pregnancy status at the time of vaccination. The time axis reflects the number of days counting from 14 days after the second dose of an mRNA COVID-19 vaccine was administered.
Table 2.
Relative Vaccine Effectiveness After 2 Doses of a Messenger RNA Coronavirus Disease 2019 Vaccine According to Pregnancy Status at the Time of Vaccination
| Vaccine | Status at Vaccination | Follow-up Time, d | Positive SARS-CoV-2 Test, No. | Unadjusted HR (95% CI) | Adjusted HR (95% CI)a |
|---|---|---|---|---|---|
| Any mRNA vaccine | Both doses while not pregnant or postpartum | 997 382 | 1500 | Ref | Ref |
| Both doses while postpartum | 278 263 | 414 | 0.98 (.88–1.09) | 1.04 (.94–1.17) | |
| Both doses while pregnant | 1 113 284 | 1531 | 0.90 (.84–.97) | 0.99 (.92–1.07) | |
| 1 dose during pregnancy and 1 dose before/after pregnancy | 526 406 | 746 | 0.95 (.87–1.03) | 1.03 (.93–1.14) | |
| BNT162b2b | Both doses while not pregnant or postpartum | 546 313 | 727 | Ref | Ref |
| Both doses while postpartum | 150 777 | 192 | 0.94 (.80–1.10) | 1.04 (.89–1.22) | |
| Both doses while pregnant | 614 760 | 772 | 0.94 (.85–1.04) | 1.04 (.93–1.16) | |
| 1 dose during pregnancy and 1 dose before/after pregnancy | 297 246 | 369 | 0.94 (.83–1.06) | 1.06 (.92–1.23) | |
| mRNA-1273b | Both doses while not pregnant or postpartum | 155 002 | 260 | Ref | Ref |
| Both doses while postpartum | 47 698 | 86 | 1.06 (.83–1.36) | 1.06 (.83–1.35) | |
| Both doses while pregnant | 179 117 | 295 | 0.98 (.83–1.15) | 0.99 (.83–1.20) | |
| 1 dose during pregnancy and 1 dose before/after pregnancy | 79 853 | 132 | 0.99 (.80–1.22) | 0.94 (.74–1.21) |
Abbreviations: CI, confidence interval; HR, hazard ratio; mRNA, messenger RNA; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Adjusted for age, education, income, region of birth, marital status, parity, various underlying chronic conditions, and number of days between dose 1 and 2, in addition to pregnancy and booster as time-varying covariates.
Homologous primary series.
Table 3.
Relative Vaccine Effectiveness After 2 Doses of a Messenger RNA Coronavirus Disease 2019 Vaccine According to Pregnancy Status at the Time of Vaccination, Stratified by Delta- and Omicron-Dominated Time Periods
| Vaccine | Status at Vaccination | Follow-up Time, d | Positive SARS-CoV-2 Test, No. | Unadjusted HR (95% CI) |
Adjusted HR (95% CI)a |
|---|---|---|---|---|---|
| Up to 31 December 2021 (Delta-dominant period) |
Both doses while not pregnant or postpartum | 732 849 | 281 | Ref | Ref |
| Both doses while postpartum | 204 720 | 73 | 0.94 (.72–1.21) | 1.01 (.78–1.30) | |
| Both doses while pregnant | 816 351 | 270 | 0.86 (.73–1.02) | 0.98 (.80–1.19) | |
| 1 dose during pregnancy and 1 dose before/after pregnancy | 384 851 | 131 | 0.88 (.71–1.08) | 1.00 (.78–1.27) | |
| From 1 January 2022 onward (Omicron-dominant period) |
Both doses while not pregnant or postpartum | 264 533 | 1219 | Ref | Ref |
| Both doses while postpartum | 73 543 | 341 | 1.01 (.89–1.14) | 1.06 (.94–1.20) | |
| Both doses while pregnant | 296 933 | 1261 | 0.92 (.85–1.00) | 1.03 (.94–1.12) | |
| 1 dose during pregnancy and 1 dose before/after pregnancy | 141 555 | 615 | 0.94 (.86–1.04) | 1.04 (.93–1.16) |
Abbreviations: CI, confidence interval; HR, hazard ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Adjusted for age, education, income, region of birth, marital status, parity, and various underlying chronic conditions, in addition to pregnancy and booster as time-varying covariates.
Relative Vaccine Effectiveness After the Booster Dose of an mRNA Vaccine According to Pregnancy Status
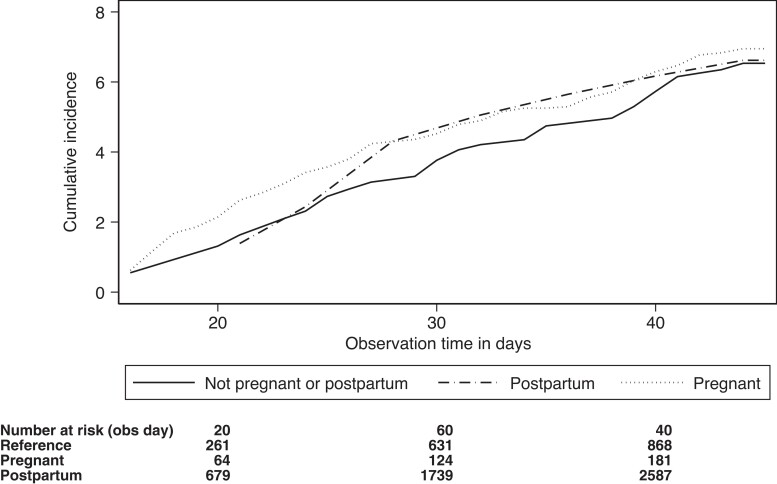
The incidence of SARS-CoV-2 per 10 000 follow-up days was 20 among women who received the booster while pregnant, 21 among women who received the booster while postpartum, and 21 among women who received the booster while neither pregnant nor postpartum. Figure 3 shows the cumulative incidence of SARS-CoV-2 infection according pregnancy status at the time of booster dose receipt. The adjusted HR for a positive test for SARS-CoV-2 was 1.12 (95% CI, .52–2.41) among women who were postpartum at the time of the booster, and 1.12 (95% CI, .84–1.84) among women who were pregnant, as compared to women who were neither pregnant nor postpartum (Table 4). The numbers were too small for analyses by vaccine product. We did not stratify these analyses according to the circulating strain because all booster vaccinations were received during the Omicron-dominant period.
Figure 3.
Cumulative incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection ≥14 days after the booster dose of a messenger RNA (mRNA) coronavirus disease 2019 vaccine according to pregnancy status at the time of vaccination. The time axis reflects the number of days from 14 days after the booster (third) vaccine dose of an mRNA vaccine against SARS-CoV-2 was administered.
Table 4.
Relative Vaccine Effectiveness After the Booster Dose of a Messenger RNA Coronavirus Disease 2019 Vaccine According to Pregnancy Status at the Time of Vaccination
| Status at Booster Vaccination | Follow-up Time, d | Positive SARS-CoV-2 Test, No. | Unadjusted HR (95% CI) | Adjusted HR (95% CI)a |
|---|---|---|---|---|
| Not pregnant or postpartum | 18 479 | 39 | Ref | Ref |
| Postpartum | 3834 | 8 | 0.98 (.46–2.10) | 1.12 (.52–2.41) |
| Pregnant | 52 820 | 103 | 0.94 (.65–1.35) | 1.24 (.84–1.84) |
Abbreviations: CI, confidence interval; HR, hazard ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Adjusted for age, education, income, region of birth, marital status, parity, various underlying chronic conditions, and time between dose 2 and 3 in days, in addition to pregnancy as time-varying covariate.
DISCUSSION
We did not observe any differences in the incidence of SARS-CoV-2 infection according to whether women received their 2-dose primary series of an mRNA COVID-19 vaccine during pregnancy or the postpartum period, as compared with women who were neither pregnant nor postpartum at the time of vaccination but had recently been pregnant. Results were similar when we evaluated the 2 mRNA vaccines separately. We also did not observe any differences in the relative effectiveness of the booster dose based on pregnancy status at the time of vaccination. These results reflect the effectiveness of vaccines against the Delta and Omicron variants of the SARS-CoV-2 virus, as they were the dominant circulating variants in the population at the time [26].
One Israeli study of 10 861 vaccinated pregnant women matched to 10 861 unvaccinated pregnant women reported a vaccine effectiveness of 96% (95% CI, 89%–100%) against any documented infection between 7 and 56 days after receiving the second dose [13]. A study of 407 vaccinated and 407 unvaccinated pregnant women from Qatar reported a vaccine effectiveness of the 2 mRNA vaccines (combined) of 88% (95% CI, 44%–97%) at least 14 days after the second dose [12]. Finally, a study of 7530 women vaccinated with BNT162b2 and 7530 unvaccinated pregnant women in Israel reported an adjusted HR for a positive PCR test for SARS-CoV-2 at 28 days or more after the first vaccine dose of 0.22 (95% CI, .11–.43), corresponding to a vaccine effectiveness of 78% (95% CI, 57%–89%) [14]. A meta-analysis of these 3 studies estimated a combined vaccine effectiveness of 90% (95% CI, 69%–96%) 7 days after the second dose of an mRNA vaccine [9]. Two of the primary studies matched vaccinated pregnant women to unvaccinated pregnant women according to demographic and clinical characteristics [13, 14], while the third study only matched for age [12]. All 3 studies were considered to have a moderate risk of bias [9]. Notably, these studies were conducted while earlier variants of the SARS-CoV-2 virus (pre-Delta) were circulating in the population [27, 28].
It has been hypothesized that vaccination during pregnancy could result in less robust immune responses due to pregnancy-induced physiological and immunological alterations [29, 30]. Although results from studies that have compared immune responses to influenza vaccination in pregnant and nonpregnant women are inconsistent—with some finding comparable levels of antibody titers and seroconversion rates and others finding both higher and lower responses in pregnant compared with nonpregnant women—estimates of influenza vaccine efficacy and effectiveness in pregnant women are similar to those of the general population [30–32]. Studies have also reported comparable immune responses to mRNA COVID-19 vaccines in pregnant and nonpregnant women of reproductive age [15, 16]. Our findings that the relative effectiveness of mRNA COVID-19 vaccines does not differ in pregnant and postpartum women is reassuring and suggests that COVID-19 vaccine effectiveness estimates derived from studies in the general adult population may inform expectations for vaccine effectiveness in pregnant populations. This is important given ongoing research and development of next-generation COVID-19 vaccines [33].
Aside from protection of pregnant women themselves, another potential benefit of vaccination during pregnancy is passive protection of infants from SARS-CoV-2 infections during the first months of life. Transplacental transfer of vaccine-derived antibodies against SARS-CoV-2 from mothers has been confirmed, and a recent study reported a 61% reduced risk of infant hospitalization for COVID-19 [34–36]. Using the Norwegian registries, we have also shown a decreased risk of SARS-CoV-2 infection during the first 4 months of life among infants born to mothers vaccinated during pregnancy [37].
Our study is unique in its population-based nature and the ability to directly compare the relative effectiveness of mRNA COVID-19 vaccines among women who were vaccinated while pregnant and those who were not during the same time interval. This avoids bias due to variations in the underlying infectious burden and circulating variants. To avoid bias due to potential confounding, we identified a comparison group of women who had been pregnant during the previous year at a similar calendar time. This comprises a group of women who had also been pregnant during the pandemic and who were of a similar age, education, income, and proportion of women with various underlying chronic diseases as the exposure groups.
Our study also has limitations. We were not able to assess the effectiveness of any non-mRNA vaccines, as the AstraZeneca vaccine (the only non-mRNA COVID-19 vaccine that was initially part of the Norwegian vaccination program) was removed in May 2021 after reports of potential links with blood coagulation disturbances [38]. We were only able to capture cases of SARS-CoV-2 infection among individuals who presented for PCR testing. This is likely to include women who had symptoms or who had strong suspicions that they might be infected due to exposure to a confirmed case. Notably, everyone with a positive antigen test was instructed to take a confirmatory PCR test during the study period. We have previously reported that pregnant women are more likely to get tested for SARS-CoV-2 compared to nonpregnant women of reproductive age [22]. As our study was conducted in a high-income country with a universal healthcare system, our results might not be generalizable to lower-resource settings.
In conclusion, pregnant women appear to derive similar protection from COVID-19 vaccination during pregnancy and the postpartum period, as compared with nonpregnant/nonpostpartum women of reproductive age. We observed similar incidence of SARS-CoV-2 infection regardless of pregnancy status at the time of vaccination. These results are reassuring, and combined with the increased risk of severe COVID-19 among pregnant women [5, 6] and the probable passive protection of the newborn [35, 37], give further support to the importance of vaccination of pregnant women.
Supplementary Material
Contributor Information
Maria C Magnus, Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway.
Siri E Håberg, Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway.
Ellen Ø Carlsen, Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway.
Jeffrey C Kwong, Public Health Ontario, Toronto, Canada; ICES, Toronto, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Canada; Centre for Vaccine Preventable Diseases, University of Toronto, Toronto, Canada; Department of Family and Community Medicine, University of Toronto, Toronto, Canada; University Health Network, Toronto, Canada.
Sarah A Buchan, Public Health Ontario, Toronto, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, Canada.
Deshayne B Fell, School of Epidemiology and Public Health, University of Ottawa, Ottawa, Canada; Children's Hospital of Eastern Ontario Research Institute, Ottawa, Canada.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Financial support. This work was supported in part by the Research Council of Norway (project number 324312 reported by S. E. H.) and through its Centres of Excellence funding scheme (project number 262700 reported by S. E. H.) and NordForsk (project number 105545 reported by M. C. M. and S. E. H.). M. C. M. has received funding from the European Research Council under the European Union's Horizon 2020 Research and Innovation Programme (grant agreement number 947684).
References
- 1. Polack FP, Thomas SJ, Kitchin N, et al. . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Voysey M, Clemens SAC, Madhi SA, et al. . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riley LE. mRNA Covid-19 vaccines in pregnant women. N Engl J Med 2021; 384:2342–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubin R. Pregnant people’s paradox—excluded from vaccine trials despite having a higher risk of COVID-19 complications. JAMA 2021; 325:1027–8. [DOI] [PubMed] [Google Scholar]
- 5. Allotey J, Fernandez S, Bonet M, et al. . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020; 370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santa S, Doku DA, Olwal CO, Brown CA, Tagoe EA, Quaye O. Paradox of COVID-19 in pregnancy: are pregnant women more protected against or at elevated risk of severe COVID-19? Future Microbiol 2022; 18:803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fell DB, Dhinsa T, Alton GD, et al. . Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA 2022; 327:1478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magnus MC, Örtqvist AK, Dahlqwist E, et al. . Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA 2022; 327:1469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prasad S, Kalafat E, Blakeway H, et al. . Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun 2022; 13:2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zavala E, Krubiner CB, Jaffe EF, et al. . Global disparities in public health guidance for the use of COVID-19 vaccines in pregnancy. BMJ Glob Health 2022; 7:e007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization . COVID-19 advice for the public: getting vaccinated. Available at:https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice. Accessed 13 April 2022.
- 12. Butt AA, Chemaitelly H, Al Khal A, et al. . SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J Clin Invest 2021; 131:e153662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dagan N, Barda N, Biron-Shental T, et al. . Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med 2021; 27:1693–5. [DOI] [PubMed] [Google Scholar]
- 14. Goldshtein I, Nevo D, Steinberg DM, et al. . Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA 2021; 326:728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collier AY, McMahan K, Yu J, et al. . Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA 2021; 325:2370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gray KJ, Bordt EA, Atyeo C, et al. . Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol 2021; 225:303.e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Norwegian Institute of Public Health . Emergency preparedness register for COVID-19 (Beredt C19). Available at:https://www.fhi.no/en/id/infectious-diseases/coronavirus/emergency-preparedness-register-for-covid-19/. Accessed 15 March 2022.
- 18. Magnus MC, Morken N-H, Wensaas K-A, Wilcox AJ, Håberg SE. Risk of miscarriage in women with chronic diseases in Norway: a registry linkage study. PLoS Med 2021; 18:e1003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Norwegian Institute of Public Health . Norwegian registry of pregnancy termination. Available at:https://www.fhi.no/en/hn/health-registries/registry-of-pregnancy-termination/. Accessed 15 January 2022.
- 20. Ammon Avalos L, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A Clin Mol Teratol 2012; 94:417–23. [DOI] [PubMed] [Google Scholar]
- 21. Mukherjee S, Velez Edwards DR, Baird DD, Savitz DA, Hartmann KE. Risk of miscarriage among black women and white women in a U.S. prospective cohort study. Am J Epidemiol 2013; 177:1271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Magnus MC, Oakley L, Gjessing HK, et al. . Pregnancy and risk of COVID-19: a Norwegian registry-linkage study. BJOG 2022; 129:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Norwegian Institute of Public Health . COVID-19 vaccination—information for health-care professionals. Available at:https://www.fhi.no/nettpub/vaksinasjonsveilederen-for-helsepersonell/vaksiner-mot-de-enkelte-sykdommene/koronavaksine/#vaksinasjon-av-gravide-og-ammende. Accessed 15 January 2022.
- 24. Norwegian Institute of Public Health . Statistics on coronavirus and covid-19. Available at:https://www.fhi.no/sv/smittsomme-sykdommer/corona/dags--og-ukerapporter/dags--og-ukerapporter-om-koronavirus/. Accessed 15 January 2022.
- 25. Norwegian Directorate of Health . Information about changes in routines for confirmatory PCR-tests and self-registering of positive home tests for COVID-19 [in Norwegian]. Available at:https://www.helsedirektoratet.no/tema/beredskap-og-krisehandtering/koronavirus/anbefalinger-og-beslutninger/Endring%20i%20rutiner%20for%20bekreftende%20PCR-test%20og%20selvregistrering%20av%20positiv%20selvtest.pdf/_/attachment/inline/e74e39b3-5993-4e8c-8c76-6df834f84aab:5adca5af69754716f27b32ac102b76f3bcdf2749/Endring%20i%20rutiner%20for%20bekreftende%20PCR-test%20og%20selvregistrering%20av%20positiv%20selvtest.pdf. Accessed 15 May 2022.
- 26. Norwegian Institute of Public Health . Risk related to the COVID-19 pandemic and the omicron variant in Norway [in Norwegian]. Available at:https://www.fhi.no/contentassets/c9e459cd7cc24991810a0d28d7803bd0/vedlegg/risikovurdering-12-01-2022.pdf. Accessed 15 February 2022.
- 27. Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 2021; 385:187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dagan N, Barda N, Kepten E, et al. . BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 384:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Omer SB. Maternal immunization. N Engl J Med 2017; 376:1256–67. [DOI] [PubMed] [Google Scholar]
- 30. Abu-Raya B, Maertens K, Edwards KM, et al. . Global perspectives on immunization during pregnancy and priorities for future research and development: an international consensus statement. Front Immunol 2020; 11:1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bansal A, Trieu M-C, Mohn KGI, Cox RJ. Safety, immunogenicity, efficacy and effectiveness of inactivated influenza vaccines in healthy pregnant women and children under 5 years: an evidence-based clinical review. Front Immunol 2021; 12:744774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Regan AK, Munoz FM. Efficacy and safety of influenza vaccination during pregnancy: realizing the potential of maternal influenza immunization. Expert Rev Vaccines 2021; 20:649–60. [DOI] [PubMed] [Google Scholar]
- 33. Nohynek H, Wilder-Smith A. Does the world still need new Covid-19 vaccines? N Engl J Med 2022; 386:2140–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flannery DD, Gouma S, Dhudasia MB, et al. . Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr 2021; 175:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Halasa NB, Olson SM, Staat MA, et al. . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. . Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study. Clin Microbiol Infect 2022; 28:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carlsen EØ, Magnus MC, Oakley L, et al. . Association of COVID-19 vaccination during pregnancy with incidence of SARS-CoV-2 infection in infants. JAMA Intern Med 2022; 182:825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Norwegian Institute of Public Health . Recommendation regarding use of the AstraZeneca vaccine [in Norwegian]. Available at:https://www.fhi.no/contentassets/3596efb4a1064c9f9c7c9e3f68ec481f/2021_04_14-anbefalingsnotat-oppdrag-21.pdf. Accessed 1 April 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.