Abstract
The N1, P2, and P3 event-related potentials (ERPs) are impaired in first-episode schizophrenia (FESz). Reduced pitch-deviant mismatch negativity (MMN) is present in chronic schizophrenia but not FESz. We examined effect sizes of, and correlations between, N1, P2, P3, and MMN in 106 FESz and 114 matched psychiatrically well controls to determine which ERPs maximally differentiated groups, and whether late sensory/perceptual deficits (N1, P2) affected preattentive memory (MMN) and conscious attention (P3). Furthermore, we compared hallucinators and nonhallucinators within FESz. Participants completed 1 of 3 oddball tasks, silently counting target tones among standard tones. Sixty-seven FESz and 72 matched participants also completed pitch-deviant MMN testing. Measures were z-scored from task appropriate controls before merging samples. Mean z-scores for N1, P2, and P3 were significantly abnormal in FESz, while pitch-deviant MMN was not. N1 showed the largest deficit (z = 0.53), and only N1 was smaller in hallucinators (n = 71) than nonhallucinators (n = 27). Among all participants, early sensory processing (N1, P2) correlated with later cognitive processing (P3), and P2 and P3 also correlated with automatic preattentive memory (pitch-deviant MMN). In well individuals, N1 was associated with MMN. These data are consistent with bottom-up sensory/perceptual processes affecting more cognitive processes. However, N1 and MMN were not associated in FESz, suggesting different auditory cortex physiology underlie these ERPs, which is differentially affected in FESz. Larger P2 and P3 with greater estimated premorbid intellect in patients indicate a possible neuroprotective effect of intellect in FESz.
Keywords: first-episode schizophrenia, N1, P2, P3, MMN, event-related potentials
Introduction
Individuals with schizophrenia have widespread impairments in auditory analysis along the information processing stream, spanning thalamic,1 sensory cortical,2,3 and secondary cortical areas,4,5 as well as analysis of auditory information across distributed polymodal circuits.6 These deficits are typically examined through high-temporal resolution neurophysiological measures of event-related potentials (ERPs) extracted from the electroencephalogram (EEG), or event-related magnetic fields (ERFs) extracted from the magnetoencephalogram (MEG). Various ERPs and ERFs have been associated with symptoms in schizophrenia, typically via correlation with symptom severity, with variable results. Of particular interest are relationships with auditory hallucinations, typically occurring in ~70% of individuals with schizophrenia, and with thought disorder, another prominent symptom associated with temporal lobe (containing auditory cortex) dysfunction. The extent of dysfunction along the auditory information-processing hierarchy early in disease course, namely at the first episode of, or first hospitalization for, presumptive schizophrenia is not well known. In addition, associations between the various neurophysiological measures and symptoms are not well described.
The auditory P3 ERP in response to actively detected target tones presented rarely among standard tones is one of the most often measured physiological responses in chronically ill schizophrenia subjects and is robustly reduced.7–9 Salisbury et al10 and Hirayasu et al11 were the first to report reduced P3 in first-episode schizophrenia patients (FESz). Salisbury et al10 compared relatively small groups of well-matched subjects (14 per group) comprising FESz, first hospitalized affective psychosis patients, and controls. P3 was reduced in FESz, with bipolar psychosis intermediate but not significantly different from controls. Hirayasu et al11 independently demonstrated similar overall P3 reduction in a study of 45 drug-naive and 56 previously treated but currently drug free patients versus controls. Twenty-seven of the drug-naive and 20 of the previously treated patients were at or within one year of their first hospitalization (a typical definition of first episode). FESz were as equally reduced as chronically ill patients. Subsequently, many other groups have reported reduced auditory P3 in FESz.12–19 Overall reductions of auditory P3 are present in FESz, indicating low-level cognitive impairments early in disease course. Furthermore, several more recent studies have demonstrated P3 is reduced in individuals at clinical high risk for psychosis (CHR), indicating the cognitive processes indexed by P3 are likely impaired prior to psychosis onset. Thus, P3 reduction has been proposed as a biomarker of incipient schizophrenia that may be useful in detection of true prodromal cases (~20%) among CHR individuals. However, P3 reductions appear to be associated with general psychopathology and are unlikely to be pathognomonic for schizophrenia or psychosis. For example, Van Tricht et al20 found overall reduced P3 in CHR that transitioned to psychosis, but P3 was also reduced in CHR that did not transition to psychosis. Although the P3 reduction was largest in CHR that transitioned, the pathophysiology appears to span psychopathology. Similarly, Özgürdal et al21 found reduced P3 in the total CHR sample, although actual transition to psychosis was not assessed.
In terms of symptom correlations, P3 over left temporal areas has been associated with thought disorder22–24 and overall positive symptoms.25–27 There are relatively few reports of P3 and hallucinations. Some report P3 is equally reduced in hallucinators and nonhallucinators,28 or that P3 is not correlated with hallucinations,6 although one report testing patients at remission reported that P3 frontal component improvement was associated with a reduction in hallucinations.29 Symptoms typically do not correlate reliably with P3 in FESz.
The earlier N1 and P2 ERPs are also reduced in long-term schizophrenia12,30–33 (see Rosburg et al34 for N1 review). Brown et al12 and Salisbury et al5 reported reduced N1 in FESz to standard tones from a P3 oddball task. Some report no P2 reductions to standard tones on an oddball task in FESz33 whereas others have.5 N1 has also attracted attention as a potential biomarker of schizophrenia prior to the emergence of psychosis. N1 and P2 appear to be reduced in CHR, but it is not known if they are pathognomonic for schizophrenia or psychosis, or if, like P3, they index psychopathology in general. For example, despite finding no N1 or P2 reduction at baseline,20 follow-up of a larger samples revealed progressive reduction in N1 among CHR individuals that transitioned to psychosis.35
Regarding hallucinations, Hubl et al36 reported smaller N1 in individuals during periods with hallucinations compared to periods without hallucinations. To our knowledge, there are no reports of auditory N1 in hallucinators versus nonhallucinators in the literature.
Finally, the mismatch negativity (MMN) is a small negative-going potential elicited by rare deviant stimuli elicited outside the focus of attention (unlike P3) that is robustly reduced in long-term schizophrenia.37–40 Salisbury et al40 were the first to report a lack of MMN reduction in FESz. Despite some reports of reduced duration MMN in FESz, a recent meta-analysis41 showed no impairment of pitch MMN and a modest reduction of duration MMN at first episode. Despite some initial reports of substantially reduced duration MMN in CHR individuals that was more marked in individuals that transitioned to psychosis,42,43 more recent work has failed to detect large MMN reductions in CHR individuals, even those that transitioned to psychosis, suggesting it is not a viable biomarker for disease presence prior to the emergence of psychosis.44
Regarding hallucinations, Fisher and colleagues45,46 noted associations between MMN and the severity of auditory hallucinations, but the majority of studies have not reported such correlations. Directly comparing hallucinators and nonhallucinators on MMN to left or right location deviants, Perrin et al47 reported no difference in MMN to left-side deviants, but paradoxically preserved MMN in hallucinators to right-side deviants. MMN did not correlate with thought disorder.
Thus, deficits in N1, P2, P3, and MMN have been reported in long-term schizophrenia, and are reliably reduced with medium to large effect sizes. The effects in FESz are less well studied, but reductions appear to be present for N1, P2, and P3. The associations with hallucinations as measured by correlations is variable in both disease stages. One possibility is that there may be a qualitative rather than quantitative relationship between hallucinations (and presumably underlying auditory cortex dysfunction) and auditory ERPs. That is, hallucinators may be relatively impaired compared to nonhallucinators, regardless of the severity of their hallucinations.
The associations between auditory ERPs are also little studied. With regard to abnormalities in the information processing stream, it is reasonable to assume that deficits in earlier stages should impact later stages of processing. However, there are few findings in that regard. To address these questions, namely how impaired N1, P2, P3, and MMN are in FESz, how the different ERP amplitudes correlate within individuals, and how they relate to the presence of auditory hallucinations, we analyzed data from over 100 FESz and over 100 matched controls, representing the largest sample of FESz to date.
Method
Participants were recruited over a 20-year period from 1993 through 2012. Subjects had no history of a learning disability, including dyslexia, special education, childhood treatment for attention deficit disorder/attention deficit hyperactivity disorder, any infectious or neurological disease affecting the central nervous system, any loss of consciousness >20 minutes and/or traumatic brain injury with sequelae, electroconvulsive therapy, drug or alcohol detox or dependence within the past 5 years, intravenous drug abuse ever, or seizure disorder. Subjects had a minimum ninth grade education and an estimated IQ > 85. All subjects had normal hearing as assessed with audiometry, defined as within 30 dB nHL (normal hearing level), no more than 15 dB difference between ears at 500, 1000, and 1500 Hz. A staggered method of ascending limits was used in 5-dB steps (begin at 0 nHL, down 1 step, up 2, down 1, up 2, etc, until detection, then down 3, repeat procedure until 3 hits at a specific intensity).
Patients between 18 and 55 years of age were recruited from consecutive inpatient admissions at a private psychiatric facility at their first hospitalization for psychosis or less than 1 year from their first inpatient admission for psychosis. All patients received a research diagnosis based on the SCID P (Structured Clinical Interview for DSM-IV Disorders–Patients) interview48 and chart review. (Approximately 50% of the first hospitalized psychosis subjects received follow-up diagnoses.) FESz included schizophrenia, schizophreniform disorder, and schizoaffective disorder. Unless patients refused medication, all were acutely medicated for therapeutic reasons. Patients were tested usually within 2 weeks of any lifetime exposure to antipsychotic medications. Control subjects were recruited from newspaper advertisements and were screened using the SCID NP49 and SCID II.50 No control subject had an Axis I psychiatric disorder in a first degree relative by report.
A total of 108 FESz and 173 psychiatrically well control subjects were tested. Subsequent culling was performed to group match age, gender, handedness, and parental socioeconomic status (PSES). Generally, this entailed sorting the healthy control group by matching variables and dropping those who matched least across matching variables until groups did not differ. Three different stimulus delivery systems were used to collect data over this long period of time. For the first 5 years stimuli were generated and presented with a Neuroscience stimulator. Sixty-seven of the subjects underwent this testing (System 1). For the second 5 years, stimuli were generated and presented using Neuroscan STIM software. Ninety-eight of the subjects underwent this testing (System 2). For both systems, subjects silently counted binaurally presented target tones (97 dB SPL, 1.5 kHz tones, 50 ms duration, 10 ms rise/fall, 15% of trials) among standard tones (97 dB, 1 kHz). Tone intensities were verified with a soundmeter. There were 200 tones presented in total; 170 standards and 30 targets. Tones were presented every 1.2 seconds on average with a jittered ISI. The major difference in paradigms was that the Neuroscience System 1 presented tones against a background of 70 dB white noise mask for extraneous room sounds, but the Neuroscan System 2 did not generate white noise. Psychophysically, then, the Neuroscan System 2 stimuli were perceived as louder. For these 165 participants, a 32-channel EEG system was used. EEG activity was recorded from the scalp through 28 tin electrodes in preconfigured caps (ElectroCap International, Eaton, OH) using Neuroscience amplifiers and Neuroscan Acquire software. Electrode sites included all 10-20 sites excluding T1/2, and including Oz; FTC1/2; TCP1/2, PO1/2; and CP1/2. Linked earlobes were the reference, the forehead was ground. Two electrodes located medially to the right eye, one above and one below, were used to monitor vertical eye movements and blinks (bipolar recording). Electrodes placed at the outer canthi of the eyes were used to monitor horizontal eye movements (bipolar recording). All electrode impedances were below 5 kOhm, and the ears were matched within 1 kohm. The EEG amplifier bandpass was 0.15 (6 dB/octave rolloff) to 40 Hz (36 dB/octave rolloff).
A total of 115 of these participants also underwent MMN testing. Subjects were presented 1600 binaural tone pips (3/second) comprising 1520 standards (75 dB, 1 kHz, 100 ms duration, 10 ms rise/fall) and 80 pitch-deviants (75 dB, 1.2 kHz, 100 ms duration, 10 ms rise/fall). Tone intensities were verified with a soundmeter. Participants performed an asynchronous visual checkerboard reversal detection task and were instructed to ignore the tones.
For the last 10 years, stimuli were presented using Superlab Pro software at 72 dB with no background masking noise (System 3). Tone intensities were verified with a soundmeter. Otherwise, stimulus and task parameters were the same, with the exception that 400 tones were presented. A total of 116 participants were tested on this system. These data were recorded from 60 scalp sites and the nose tip using a 64-channel cap (custom designed Electro-Cap International sintered Ag-AgCl caps using the 10-10 system). Activity was recorded continuously using SynAmps and Scan Acquire (Neuroscan/Compumedics USA). The right mastoid served as the recording reference, except for 2 bipolar electro-oculogram channels. Two electrodes medial to the right eye, one above and one below, monitored vertical eye movements and blinks. Electrodes at the outer canthi of the eyes monitored horizontal eye movements. The forehead served as ground. Electrode impedances were below 5 kOhm. The EEG bandpass was 0.10 (6 dB/octave roll-off) to 100 Hz (24 dB/octave rolloff). EEG was digitized at 500 Hz. N1 and P2 were measured from the oddball task standard stimuli, and P3 was measured from the oddball task target stimuli.
Eighty-two of these participants also underwent MMN testing. The task was similar to the 32-channel system MMN task with an asynchronous visual checkerboard detection task. Tone pips were present 3/second; however, standard tones were 50 ms duration (75 dB, 1 kHz, 50 ms duration, 10 ms rise/fall), and both pitch-deviants (75 dB, 1.2 kHz, 50 ms duration, 10 ms rise/fall) and duration-deviants (75 dB, 1 kHz, 100 ms duration, 10 ms rise/fall) were presented 10% of the time. For both MMN tasks, MMN was visualized by subtracting the ERP waveform to standards from the waveform to deviants.
To analyze data across the different systems and tasks, matched groups were constructed for each system. Then, for each subsample, z-scores were calculated for each participant based on the control means and standard deviations. This thus expressed FESz samples data as deviations from the control means relative to standard deviations, a prima facie measure of effect size. Data were measured at the common site across EEG systems with the largest ERPs: Cz for N1 and P2, Pz for P3, and Fz for MMN. Amplitudes reflect mean voltages over windows centered on the group grand average peak latencies, with window size based on our previous initial published reports. For N1 and P2, 10-ms intervals were used. For P300, 25-ms intervals were used. For MMN, 100-ms intervals were used. Because the majority of participants contributing to MMN only received pitch-deviants, duration deviants are not reported here. Finally, z-scores for each ERP were analyzed in the entire groups. This resulted in 106 FESz and 114 well-matched psychiatrically well participants for the oddball task ERPs, and 67 FESz and 72 controls that also had MMN testing. Demographic data are presented in Table 1 for each system subsample and the entire sample. Clinical data are presented for the entire FESz sample in Table 2.
Table 1.
Demographic Information.
| System 1 Subsample |
System 2 Subsample |
System 3 Subsample |
All Subjects |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FESz | HC | P | FESz | HC | P | FESz | HC | P | FESz | HC | P | |
| N (male/female) | 32 (26/6) | 34 (27/7) | .85 | 25 (22/3) | 33 (28/5) | .73 | 49 (34/15) | 47 (33/14) | .93 | 106 (82/24) | 114 (88/26) | .98 |
| Age, y, mean (SD) | 28.7 (7.2) | 27.6 (6.4) | .52 | 23.5 (5.6) | 23.7 (4.2) | .91 | 23.3 (6.1) | 24.9 (7.7) | .25 | 25.0 (6.8) | 25.3 (6.6) | .67 |
| PSES, mean (SD) | 1.9 (1.1) | 1.7 (1.0) | .29 | 2.1 (1.1) | 1.8 (1.1) | .37 | 1.9 (1.6) | 1.8 (1.0) | .94 | 1.9 (1.4) | 1.8 (1.0) | .32 |
| Info, mean (SD) | 11.5 (3.0) | 11.6 (1.8) | .84 | 12.1 (2.8) | 12.8 (1.9) | .25 | 12.4 (2.2) | 12.6 (1.8) | .60 | 12.0 (2.6) | 12.4 (1.9) | .26 |
Abbreviations: FESz, first-episode schizophrenia; HC, healthy controls; PSES, parental socioeconomic status; Info, scaled score on WAIS (Wechsler Adult Intelligence Scale) Information subtest.
Table 2.
Clinical Information.
| Mean (SD) | |
|---|---|
| BPRS total | 39.4 (10.0) |
| Hostility_suspiciousness | 6.9 (3.2) |
| Emotional_withdrawal | 7.5 (3.7) |
| Thinking_disturbance | 8.9 (3.5) |
| Hallucinations | 3.3 (1.9) |
| Medication (CPZ equivalents) | 268.7 (268.9) |
| Illness duration (months) | 8.1 (24.7) |
| First hospitalization to EEG (weeks) | 9.3 (23.9) |
Abbreviations: BPRS, Brief Psychiatric Rating Scale; CPZ, chlorpromazine.
Demographic data were compared between groups with t tests, with the exception of gender distributions, which utilized chi-square tests. Qualitative comparison of ERP amplitudes between hallucinators and nonhallucinators utilized a t test. For quantitative analysis of associations between ERP amplitudes and symptom severity, correlations were performed using Pearson’s r examining Brief Psychiatric Rating Scale (BPRS) total, hostility_suspiciousness, emotional_withdrawal, and thinking_disturbance factors, and the hallucination item. Significance was attained at P ≤ .05.
Results
Event-Related potentials
N1 was significantly reduced at Cz in FESz, z = 0.53, t(218) = 4.06, P < .001 (Figure 1, panel A). P2 was also significantly reduced in FESz, z = 0.39, t(218) = 3.09, P = .002 (Figure 1, panel A). P3 was reduced in FESz, z = 0.46, t(218) = 3.54, P < .001 (Figure 1, panel B). Pitch-deviant MMN was not significantly reduced in FESz, z = 0.07, t(137) = 0.42, P > .67, Figure 1, panel C).
Figure 1.
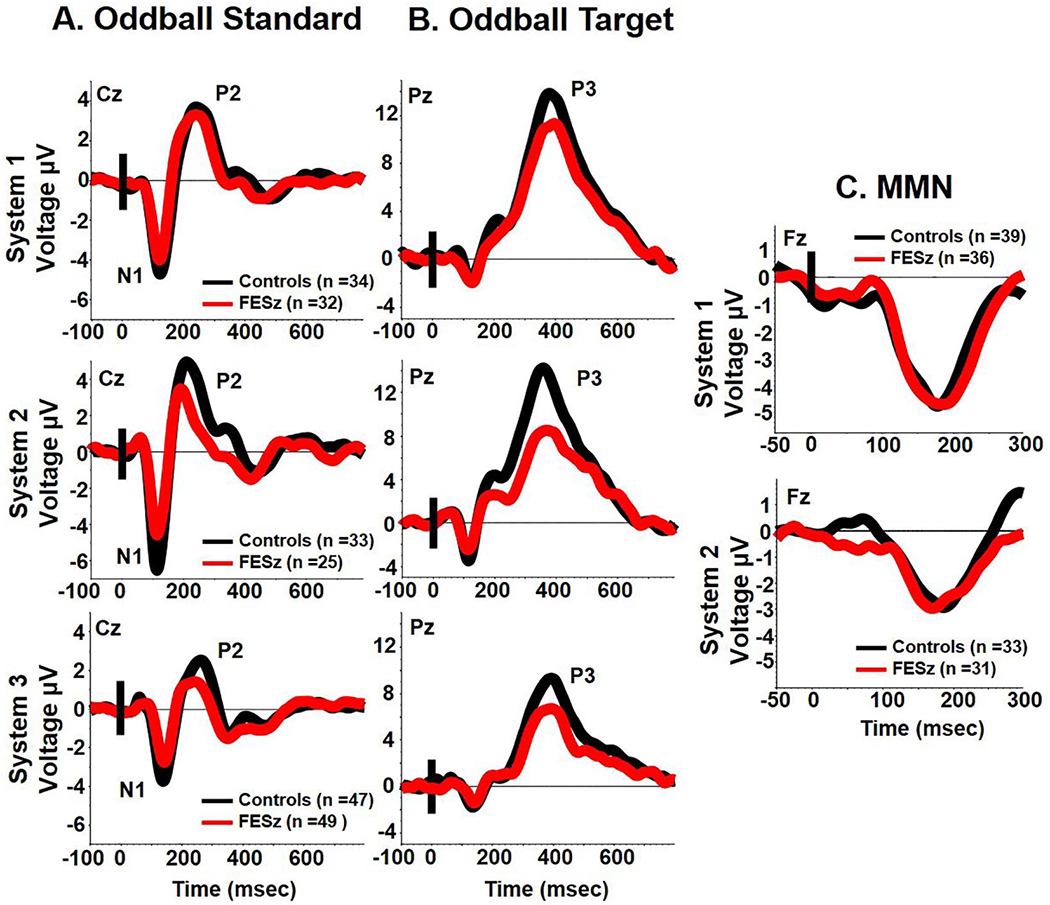
Grand averages for standard (panel A) and target tones (panel B) on the oddball task and pitch-deviant mismatch negativity (MMN) (panel C) for each system.
ERPs and Symptoms
For qualitative analyses among FESz that reported hallucinations or appeared to be responding to internal stimuli (n = 71) compared with nonhallucinators (n = 27, archived symptom data were unavailable for 8 FESz), only N1 was reduced. Hallucinators showed marked N1 reductions (z = 0.67) relative to nonhallucinators, z = 0.18, t(96) = 2.35, P = .021 (Figure 2). Nonhallucinators were not different from controls, t(139) = 0.85, P = .396 (Figure 2). The other auditory ERPs (P2, P3, and pitch-deviant MMN) did not differ between hallucinating and nonhallucinating FESz subgroups. Quantitative correlations between ERPs and symptom severity revealed only an association between smaller (more positive) N1 and greater BPRS total symptoms (r = 0.24, P = .02, Figure 3). No ERP was associated with hallucination severity.
Figure 2.
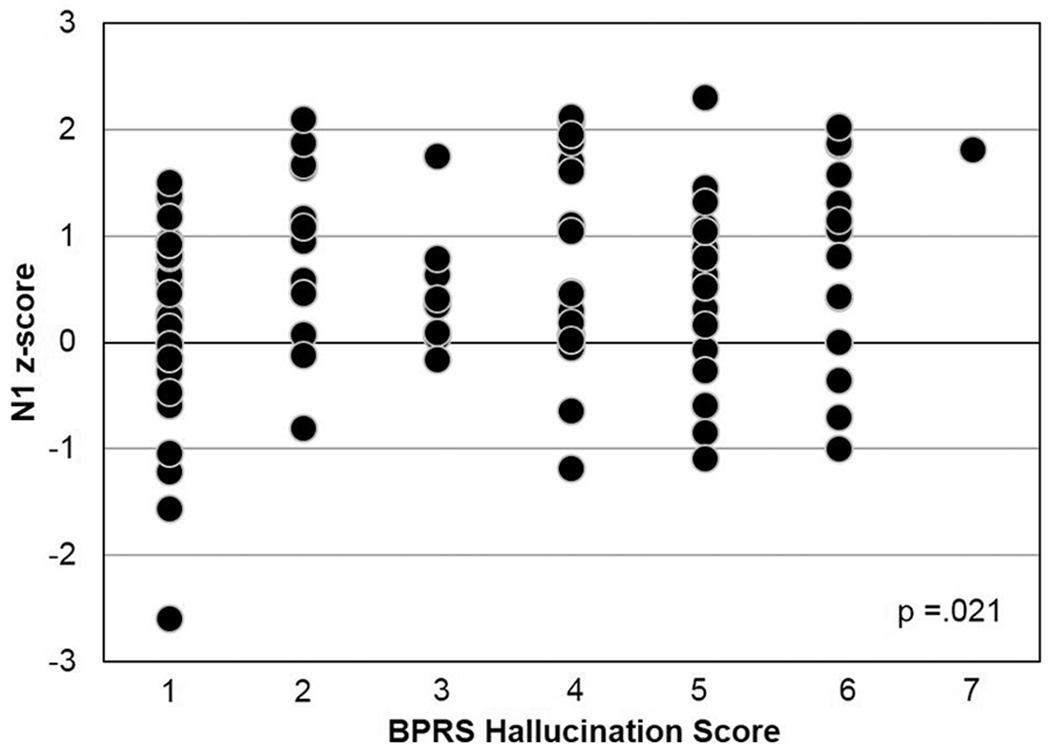
N1 is qualitatively impaired in first-episode schizophrenia (FESz) hallucinators, but nonhallucinators have N1 amplitudes within normal limits.
Figure 3.
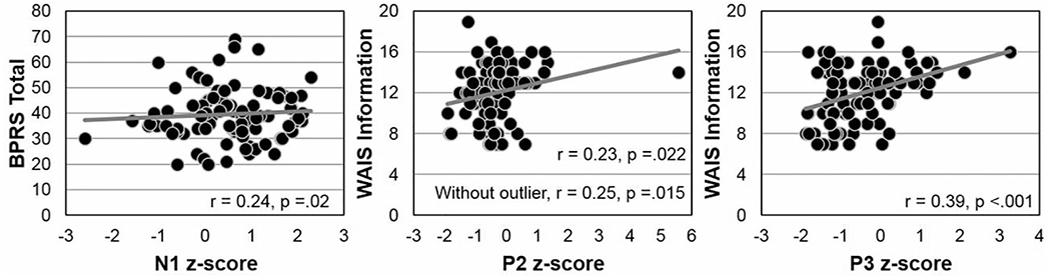
Correlations between N1 and Brief Psychiatric Rating Scale (BPRS), and between P2 and P3 and estimated premorbid IQ via the Wechsler Adult Intelligence Scale (WAIS) Information subtest.
ERPs and Estimated Premorbid IQ
Premorbid IQ was estimated by scaled scores of the WAIS Information subtest. Among psychiatrically well individuals, there were no significant correlations between WAIS Information scaled scores and any z-scored ERP. Among FESz, however, estimated premorbid IQ was directly associated with P2 (r = 0.23, P = .022) and P3 (r = 0.39, P < .001) amplitudes (Figure 3).
Interrelationships Between ERPs
Within FESz, N1 amplitudes were directly associated with P2 to standards (r = −0.27, P = .006) and P3 to oddballs (r = −0.29, P = .003), but not MMN to pitch-deviants (r = 0.20, P = .102). In addition to its association with N1, P2 was directly associated with P3 (r = 0.40, P < .001) and with MMN (r = −0.26, P = .031). In addition to its associations with N1 and P2, P3 was associated with MMN (r = −0.26, P = .034). For all ERPs, larger amplitudes (whether positive- or negative-going potentials) were associated with larger amplitudes of other ERPs (regardless of polarity).
Within psychiatrically well controls, N1 amplitudes were directly associated with P2 to standards (r = −0.26, P = .005), marginally with P3 to oddballs (r = −0.18, P = .053), and with MMN to pitch-deviants (r = 0.54, P < .001). In addition to its association with N1, P2 was directly associated with P3 (r = 0.33, P < .001) and marginally associated with MMN (r = −0.22, P = .064). In addition to its associations with N1 and P2, P3 was directly associated with MMN (r = −0.35, P = .003). For all ERPs, larger amplitudes (whether positive- or negative-going potentials) were associated with larger amplitudes of other ERPs (regardless of polarity).
The pattern of associations between ERP amplitudes was largely similar in the groups with the exception of the correlations between N1 to standards on the oddball task with P3 to targets on the oddball task and pitch-deviant MMN. The association between N1 and P3 did not significantly differ between groups. However, the association between N1 and MMN was significantly smaller in FESz than in psychiatrically well participants (r to z transform = −2.3, P = .02). Like the N1 z-score amplitude in nonhallucinators not differing from psychiatrically well comparison participants, N1 and MMN were correlated in nonhallucinators (r = 0.54, P = .029) as in controls, but were not in hallucinators (r = 0.06, P = .72).
Discussion
This article includes the largest sample of FESz reported to date on several ERP measures of auditory processing. The aim of this study was to determine, in this large sample, which ERPs maximally differentiated FESz from psychiatrically well controls, to determine if stable relationships between ERPs and symptoms and premorbid IQ were present, and to determine the degree to which impairments in ERPs were interrelated.
In an auditory oddball task, N1 and P2 to standard tones and P3 to target tones were reduced in FESz. Cohen’s d, a measure of effect size calculated by subtracting the means of two samples and dividing by the pooled variance, can be approximated by the z-score mean of FESz, as they were computed using the mean and standard deviation of the psychiatrically well individuals. There were small to medium effect sizes for P2 (z = 0.39) and P3 (z = 0.46) and a medium effect size for N1 (z = 0.53). Thus, reductions in these ERPs may serve as biomarkers of disease presence. A related question is whether they may also serve as biomarkers of disease risk in the prodrome. Each of these ERPs may prove useful for calculating risk for conversion, but even for the largest effect size in FESz of Cohen’s d of 0.53 for N1, 79% of the group distributions will overlap. For clinical utility, larger effect sizes would be optimal, on the order of 1.5 to 2 SDs. Combining the various ERPs will not improve the risk calculation much, because N1, P2, and P3 are somewhat correlated in FESz and would not provide much unique variance. Still, the reductions of these ERPs are reliable, and provide clues as to the underlying pathology in emerging psychosis. Pitch-deviant MMN, by contrast, was not reduced in FESz, and is not a reasonable candidate for a biomarker of disease presence.
N1 showed the largest z-score impairment in FESz and was associated with a large impairment in hallucinators (z = 0.67), who were significantly reduced by comparison with nonhallucinators. This is the first report of N1 reductions specific to hallucinators in FESz. N1 appears to be uniquely associated with auditory system pathology underlying hallucinations in FESz, but this effect is qualitative rather than quantitative; all hallucinators appear to be equally reduced (Figure 2). P2, P3, and MMN did not differentiate hallucinators and nonhallucinators, nor were there quantitative correlations between any ERP amplitude and symptom severity. N1 was associated indirectly with overall symptom severity, but this small-sized association is likely trivial. Still, these data suggest that N1 may be especially sensitive to auditory pathophysiology near psychosis onset and may have particular merit to provide clues to underlying pathology related to hallucinations.
ERPs were interrelated, such that early sensory/perceptual processing indexed by N1 and P2 were associated with each other and with P3 to target tones on the oddball task. P2 and P3 were also associated with the amplitude of pitch-deviant MMN. These associations did not meaningfully differ between FESz and psychiatrically well control participants. However, the pattern of N1 associations with pitch-deviant MMN did differ between groups, with a lack of association in FESz. This suggests that the cortical substrate of pitch-deviant MMN, which was not impaired in FESz, is not the same as the cortical substrate of N1, which was reduced in FESz. Thus, N1 deficits and MMN deficits (which are presumed to develop with psychosis course51,52 may be sensitive to different patterns of progressive pathology associated with the emergence of psychosis and provide differential information about underlying biological mechanisms of pathology. Future studies should aim to definitively determine the cortical sources and underlying pathological circuit architecture giving rise to N1 and MMN to provide clues to potential novel therapeutic pharmacologic targets.
Finally, in FESz, better premorbid IQ was associated weakly with a larger P2 to standard stimuli (an association that remained when the outlier was removed), and more markedly with a larger P3 to target oddball stimuli. The small effect size of the P2 correlation suggests it may be discounted, but the greater effect size of the correlation between estimated premorbid IQ and P3 suggests it is meaningful. Robust decrements of P3 are observed in long-term schizophrenia and in FESz, and psychosis has an immediate impact on intellectual functioning. To the extent that reduced P3 reflects impaired distributed cortical processing and structural pathology of some cortical areas, we speculate that better premorbid IQ may confer some neuroprotective effect during early psychosis.53
Although this article is highly powered, several caveats must be considered. Different systems were used across subsamples. This may have introduced variations in ERPs. These variations were mitigated by z-scoring each sample for each system against matched comparison subjects as system specific normative values. Different task parameters were used on each system. This may modify the degree of group differences based on stimulus parameters. Such effects may be partially mitigated by z-scoring, and yet may also remain in the merged data. Because different montages were used across systems, full topographic analyses could not be performed. This may miss important information about regional abnormalities, as analyses were reduced to a single electrode site. We did not have more than 1 sample with duration-deviant MMN so could not perform a multisample analysis. As duration-deviant MMN may have a larger effect size at first psychotic episode,41,54 we may be missing an important effect. We did not adjust for multiple comparisons and correlations. We believe this is mitigated by the large sample sizes, which should better approximate the true population. Still, caution should be used in interpreting the results, and replication is needed. We are currently collecting a large replication sample on these measures.
In summary, N1, P2, and P3 but not pitch-deviant MMN were reduced in FESz with small to medium effect sizes. N1 showed weak correlations with overall BPRS scores and was significantly reduced in hallucinators compared to nonhallucinators as well as psychiatrically well controls. Furthermore, unlike in controls, N1 was not correlated with pitch-deviant MMN in hallucinating FESz. These data suggest that widespread deficits of auditory processing exist in FESz along the information processing hierarchy. Furthermore, early sensory/perceptual processing influences later active deviance detection (P3) in both groups and is related to automatic deviance detection (MMN) in healthy individuals but not in FESz. The latter finding suggests that N1 and MMN are sensitive to different underlying pathology. We speculate that N1 and MMN have different cortical generators, with the N1 generators being impaired at first psychosis and specifically related to hallucinations, while the pitch-deviant MMN generators are not. This may reflect pathology in different cortical areas or deficits in different cortical layers within circuits in the same cortical column. Each ERP may provide important clues to underlying pathology in emerging psychosis. Last, better intellectual function is reflected in more intact brain processing of simple oddball targets, a low-level cognitive operation, consistent with the notion that higher IQ confers some neuroprotective effect in emerging psychosis.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Department of Veterans Affairs (Merit Award, Schizophrenia Center Award, Middleton Award to RWM), by the National Institute of Health (R01 MH58704 to DFS; RO1 MH01110 and RO1 MH50747 to MES; R01 MH40799, R01 MH052807, P50 MH080272 to RWM), the MIND foundation (RWM), and NARSAD (DFS).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Full-color figures are available online at journals.sagepub.com/home/eeg
References
- 1.Leavitt VM, Molholm S, Ritter W, Shpaner M, Foxe JJ. Auditory processing in schizophrenia during the middle latency period (10-50 ms): high-density electrical mapping and source analysis reveal subcortical antecedents to early cortical deficits. J Psychiatry Neurosci. 2007;32:339–353. [PMC free article] [PubMed] [Google Scholar]
- 2.Erwin RJ, Shtasel D, Gur RE. Effects of medication history on midlatency auditory evoked responses in schizophrenia. Schizophr Res. 1994;11:251–258. [DOI] [PubMed] [Google Scholar]
- 3.Huang MX, Edgar JC, Thoma RJ, et al. Predicting EEG responses using MEG sources in superior temporal gyrus reveals source asynchrony in patients with schizophrenia. Clin Neurophysiol. 2003;114:835–850. [DOI] [PubMed] [Google Scholar]
- 4.Hanlon FM, Miller GA, Thoma RJ, et al. Distinct M50 and M100 auditory gating deficits in schizophrenia. Psychophysiology. 2005;42:417–427. [DOI] [PubMed] [Google Scholar]
- 5.Salisbury DF, Collins KC, McCarley RW. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophr Bull. 2010;36:991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salisbury DF, Shenton ME, McCarley RW. P300 topography differs in schizophrenia and manic psychosis. Biol Psychiatry. 1999;45:98–106. [DOI] [PubMed] [Google Scholar]
- 7.Roth WT, Cannon EH. Some features of the auditory evoked response in schizophrenics. Arch Gen Psychiatry. 1972;27:466–471. [DOI] [PubMed] [Google Scholar]
- 8.Duncan CC. Event-related brain potentials: a window on information processing in schizophrenia. Schizophr Bull. 1988;14:199–203. [DOI] [PubMed] [Google Scholar]
- 9.Ford JM, White PM, Csernansky JG, Faustman WO, Roth WT, Pfefferbaum A. ERPs in schizophrenia: effects of antipsychotic medication. Biol Psychiatry. 1994;36:153–170. [DOI] [PubMed] [Google Scholar]
- 10.Salisbury DF, Shenton ME, Sherwood AR, et al. First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiatry. 1998;55:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirayasu Y, Asato N, Ohta H, Hokama H, Arakaki H, Ogura C. Abnormalities of auditory event-related potentials in schizophrenia prior to treatment. Biol Psychiatry. 1998;43:244–253. [DOI] [PubMed] [Google Scholar]
- 12.Brown KJ, Gonsalvez CJ, Harris AW, Williams LM, Gordon E. Target and non-target ERP disturbances in first episode vs. chronic schizophrenia. Clin Neurophysiol. 2002;113:1754–1763. [DOI] [PubMed] [Google Scholar]
- 13.Demiralp T, Üçok A, Devrim M, Isoglu-Alkaç Ü, Tecer A, Polich J. N2 and P3 components of event-related potential in first-episode schizophrenic patients: scalp topography, medication, and latency effects. Psychiatry Res. 2002;111:167–179. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Hirayasu Y, Hiramatsu KI, Hokama H, Miyazato H, Ogura C. Increased rate of P300 latency prolongation with age in drug-naive and first episode schizophrenia. Clin Neurophysiol. 2003;114:2029–2035. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Hirayasu Y, Hokama H, et al. Influence of duration of untreated psychosis on auditory P300 in drug-naive and first-episode schizophrenia. Psychiatry Clin Neurosci. 2005;59:209–214. [DOI] [PubMed] [Google Scholar]
- 16.Van Der Stelt O, Lieberman JA, Belger A. Auditory P300 in high-risk, recent-onset and chronic schizophrenia. Schizophr Res. 2005;77:309–320. [DOI] [PubMed] [Google Scholar]
- 17.Devrim-Üçok M, Keskin-Ergen HY, Üçok A. Novelty P3 and P3b in first-episode schizophrenia and chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1426–1434. [DOI] [PubMed] [Google Scholar]
- 18.Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006;59:762–772. [DOI] [PubMed] [Google Scholar]
- 19.Renoult L, Prévost M, Brodeur M, et al. P300 asymmetry and positive symptom severity: a study in the early stage of a first episode of psychosis. Schizophr Res. 2007;93:366–373. [DOI] [PubMed] [Google Scholar]
- 20.van Tricht MJ, Nieman DH, Koelman JH, et al. Reduced parietal P300 amplitude is associated with an increased risk for a first psychotic episode. Biol Psychiatry. 2010;68:642–648. [DOI] [PubMed] [Google Scholar]
- 21.Özgürdal S, Gudlowski Y, Witthaus H, et al. Reduction of auditory event-related P300 amplitude in subjects with at-risk mental state for schizophrenia. Schizophr Res. 2008;105:272–278. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki Y, Maeda Y, Higashima M, et al. Reduced auditory P300 amplitude, medial temporal volume reduction and psychopathology in schizophrenia. Schizophr Res. 1997;26:107–115. [DOI] [PubMed] [Google Scholar]
- 23.Kirihara K, Araki T, Kasai K, et al. Confirmation of a relationship between reduced auditory P300 amplitude and thought disorder in schizophrenia. Schizophr Res. 2005;80:197–201. [DOI] [PubMed] [Google Scholar]
- 24.Debruille JB, Schneider-Schmid A, Dann P, King S, Laporta M, Bicu M. The correlation between positive symptoms and left temporal event-related potentials in the P300 time window is auditory specific and training sensitive. Schizophr Res. 2005;78:117–125. [DOI] [PubMed] [Google Scholar]
- 25.Higashima M, Nagasawa T, Kawasaki Y, et al. Auditory P300 amplitude as a state marker for positive symptoms in schizophrenia: cross-sectional and retrospective longitudinal studies. Schizophr Res. 2003;59:147–157. [DOI] [PubMed] [Google Scholar]
- 26.Meisenzahl EM, Frodl T, Müller D, et al. Superior temporal gyrus and P300 in schizophrenia: a combined ERP/structural magnetic resonance imaging investigation. J Psychiatr Res. 2004;38:153–162. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki Y, Sumiyoshi T, Higuchi Y, Ito T, Takeuchi M, Kurachi M. Voxel-based analysis of P300 electrophysiological topography associated with positive and negative symptoms of schizophrenia. Schizophr Res. 2007;94:164–171. [DOI] [PubMed] [Google Scholar]
- 28.Oades RD, Zerbin D, Dittmann-Balcar A, Eggers C. Auditory event-related potential (ERP) and difference-wave topography in schizophrenic patients with/without active hallucinations and delusions: a comparison with young obsessive-compulsive disorder (OCD) and healthy subjects. Int J Psychophysiol. 1996;22:185–214. [DOI] [PubMed] [Google Scholar]
- 29.Turetsky B, Colbath EA, Gur RE. P300 subcomponent abnormalities in schizophrenia: II. Longitudinal stability and relationship to symptom change. Biol Psychiatry. 1998;43:31–39. [DOI] [PubMed] [Google Scholar]
- 30.Saletu B, Itil TM, Saletu M. Auditory evoked response, EEG, and thought process in schizophrenics. Am J Psychiatry. 1971;128:336–344. [DOI] [PubMed] [Google Scholar]
- 31.Connolly JF, Manchanda R, Gruzelier JH, Hirsch SR. Pathway and hemispheric differences in the event-related potential (ERP) to monaural stimulation: a comparison of schizophrenic patients with normal controls. Biol Psychiatry. 1985;20:293–303. [DOI] [PubMed] [Google Scholar]
- 32.Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr Res. 1999;37:65–79. [DOI] [PubMed] [Google Scholar]
- 33.O’Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int J Psychophysiol. 2004;53:45–55. [DOI] [PubMed] [Google Scholar]
- 34.Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia—a critical review. Psychiatry Res. 2008;161:259–274. [DOI] [PubMed] [Google Scholar]
- 35.van Tricht MJ, Nieman DH, Koelman JH, et al. Auditory ERP components before and after transition to a first psychotic episode. Biol Psychol. 2011;87:350–357. [DOI] [PubMed] [Google Scholar]
- 36.Hubl D, Koenig T, Strik WK, Garcia LM, Dierks T. Competition for neuronal resources: how hallucinations make themselves heard. Br J Psychiatry. 2007;190:57–62. [DOI] [PubMed] [Google Scholar]
- 37.Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991;30:1059–1062. [DOI] [PubMed] [Google Scholar]
- 38.Javitt DC, Grochowski S, Shelley AM, Ritter W. Impaired mismatch negativity (MMN) generation in schizophrenia as a function of stimulus deviance, probability, and interstimulus/interdeviant interval. Electroencephalogr Clin Neurophysiol. 1998;108:143–153. [DOI] [PubMed] [Google Scholar]
- 39.Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol. 2001;42:177–194. [DOI] [PubMed] [Google Scholar]
- 40.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. [DOI] [PubMed] [Google Scholar]
- 41.Haigh SM, Coffman BA, Salisbury DF. Mismatch negativity in first-episode schizophrenia: a meta-analysis. Clin EEG Neurosci. 2017;48:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol Psychiatry. 2012;71:98–104. [DOI] [PubMed] [Google Scholar]
- 43.Bodatsch M, Ruhrmann S, Wagner M, et al. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69:959–966. [DOI] [PubMed] [Google Scholar]
- 44.Atkinson RJ, Fulham WR, Michie PT, et al. Electrophysiological, cognitive and clinical profiles of at-risk mental state: the longitudinal Minds in Transition (MinT) study. PLoS One. 2017;12:e0171657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher DJ, Labelle A, Knott VJ. The right profile: mismatch negativity in schizophrenia with and without auditory hallucinations as measured by a multi-feature paradigm. Clin Neurophysiol. 2008;119:909–921. [DOI] [PubMed] [Google Scholar]
- 46.Fisher DJ, Grant B, Smith DM, Borracci G, Labelle A, Knott VJ. Effects of auditory hallucinations on the mismatch negativity (MMN) in schizophrenia as measured by a modified “optimal” multi-feature paradigm. Int J Psychophysiol. 2011;81:245–251. [DOI] [PubMed] [Google Scholar]
- 47.Perrin MA, Kantrowitz JT, Silipo G, Dias E, Jabado O, Javitt DC. Mismatch negativity (MMN) to spatial deviants and behavioral spatial discrimination ability in the etiology of auditory verbal hallucinations and thought disorder in schizophrenia. Schizophr Res. 2018;191:140–147. [DOI] [PubMed] [Google Scholar]
- 48.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 1998. [Google Scholar]
- 49.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Non-Patient Edition (SCID I/NP). New York, NY: Biometrics Research, New York State Psychiatric Institute; 1998. [Google Scholar]
- 50.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-I V Axis II Disorders (SCID II). Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 51.Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salisbury DF, Polizzotto NR, Nestor PG, Haigh SM, Koehler J, McCarley RW. Pitch and duration mismatch negativity and premorbid intellect in the first hospitalized schizophrenia spectrum. Schizophr Bull. 2017;43:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leeson VC, Sharma P, Harrison M, Ron MA, Barnes TRE, Joyce EM. IQ trajectory, cognitive reserve and clinical outcome following a first-episode of psychosis: a three year longitudinal study. Schizophr Bull. 2009;37:768–777. doi: 10.1093/schbul/sbp143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erickson MA, Ruffle A, Gold JM. A meta-analysis of mismatch negativity in schizophrenia: from clinical risk to disease specificity and progression. Biol Psychiatry. 2016;79:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]


