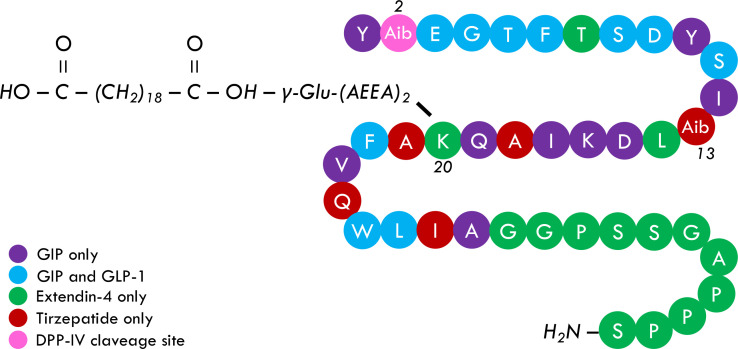
Figure 1.
Tirzepatide primary structure. TZP is a 39-peptide containing different native and not-native incretins fragments, including GIP, GIP, GLP-1, and Extendin-4 (and glucagon, not shared). Alpha aminobutyric acid is placed in positions 2 and 13 and provides TZP an intrinsic resistance to DPP-IV attack and more structural stability, respectively. A 20-carbon fatty acid, namely eicosanedioic acid, is linked to Glu. A 2xAdo unit is attached to the lysine residue in position 20 and allows TZP to be bound to albumin, consequently increasing its half-life to five days. It is currently debated whether the acylation may also increase receptor bounding stability, hypothetically affecting the pharmacological potency of TZP.