Abstract
Opioid addiction is a chronic, relapsing disorder. Whether addicted individuals are forced to abstain or they decide themselves to quit using drugs, relapse rates are high—especially upon encountering contexts and stimuli associated with prior opioid use. Rodents similarly show context- and cue-induced reinstatement of drug seeking following abstinence, and intriguingly, the neural circuits underlying these relapse-like behaviors differ when abstinence is involuntarily imposed, responding is extinguished, or animals decide themselves to cease taking drug. Here, we employ two complementary rat behavioral models of relapse-like behavior for the highly reinforcing opioid drug remifentanil, and asked whether GABAergic neurons in the ventral pallidum (VPGABA) control opioid seeking under these behavioral conditions. Specifically, we asked how chemogenetically stimulating VPGABA neurons with clozapine-N-oxide (CNO) influences the ability of contextual or discrete remifentanil-paired cues to reinstate drug seeking following either voluntary abstinence (punishment-induced; GroupPunish), or extinction training (GroupExt). In GroupPunish rats, we also chemogenetically inhibited VPGABA neurons, and examined spontaneous VP activity (Fos) during cued reinstatement. In both GroupPunish and GroupExt rats, stimulating Gq-signaling in VPGABA neurons augmented remifentanil reinstatement in a cue- and context-dependent manner. Conversely, engaging inhibitory Gi-signaling in VPGABA neurons in GroupPunish suppressed cue-induced reinstatement, and cue-triggered seeking was correlated with Fos expression in rostral, but not caudal VP. Neither stimulating nor inhibiting VPGABA neurons influenced unpunished remifentanil self-administration. We conclude that VPGABA neurons bidirectionally control opioid seeking regardless of the specific relapse model employed, highlighting their fundamental role in opioid relapse-like behavior across behavioral models, and potentially across species.
Keywords: Punishment, Extinction, Abstinence, Reinstatement, Chemogenetics, DREADDs, Remifentanil
Introduction
Opioid addiction is a disorder characterized by persistent drug use despite adverse consequences, and chronic risk of relapse after quitting. Though addicted individuals frequently quit drug use due to mounting negative consequences, they often still relapse despite their desire to remain abstinent [1–3]. In particular, exposure to drug-associated cues or contexts often elicit cravings and promote relapse [4].
Much preclinical work has examined the neural circuits of cue-, stress-, or drug-induced relapse-like behavior in rodents, especially using models involving experimenter-imposed abstinence or extinction training prior to reinstatement of drug seeking [5]. Yet these conventional models do not capture the voluntary initiation of abstinence that is typical of addicted humans seeking to control their use in the face of mounting negative life consequences—rats in these experiments have little disincentive to pursue drugs when they may be available [6, 7]. It has been argued that the presence of such disincentives to drug use might be important for preclinically modeling addiction [8–10], especially since the neural substrates underlying reinstatement differ when rats previously chose to stop taking drugs, rather than undergoing extinction training [11, 12]. Furthermore, no single rodent model captures all aspects of the human use-cessation-relapse cycle, so to maximize likelihood of translational relevance, we propose that putative addiction interventions should be tested in multiple rodent behavioral models including those optimizing human relevance [13]. We hope that by understanding the converging neural circuits underlying relapse across animal models that capture distinct features of the human disorder, we can identify more promising candidates for targeting brain-based psychiatric interventions in humans struggling to control their drug use.
Many prior rodent reinstatement studies have examined the brain substrates of relapse following experimenter-imposed homecage abstinence (such as incubation of craving), or extinction training [14–21]. Fewer studies have used models in which rodents instead voluntarily cease their drug use, for example due to delivery of punishing shocks co-administered with drug. For opioid drugs, this is partly due to the methodological consideration that the analgesic properties of opioid drugs can diminish the ability of shock to suppress drug seeking. Here, we circumvented this problem by using the short-acting, but highly reinforcing μ opioid receptor agonist remifentanil, similar to a model presented by Panlilio and colleagues [11, 22]. Since remifentanil is rapidly metabolized [23], we were able to develop a shock-based voluntary abstinence/reinstatement procedure, allowing for direct comparison of opioid reinstatement following either voluntary punishment-induced abstinence, or extinction training.
Specifically, we examined the role of ventral pallidum (VP), a brain region tightly embedded within mesocorticolimbic motivational circuits, where opioid signaling plays important roles in reward-related processes [24, 25]. Locally applied μ opioid receptor agonists in VP induce robust food intake and locomotion, and enhance pleasure-like reactions to sweet tastes [25, 26]. Systemically administered heroin or morphine decrease extracellular GABA levels in VP [27, 28], and lesioning or inactivating VP neurons diminishes high-effort responding for heroin [29], and the ability of heroin priming injections to reinstate heroin seeking following extinction training [30]. VP is also required for high-effort intake of remifentanil, since local application of an orexin receptor antagonist attenuates remifentanil motivation in both behavioral economic and cue-induced reinstatement tasks [31]. Therefore, VP is a key node in the circuits underlying the rewarding and relapse-inducing effects of addictive opioid drugs.
This said, VP is a heterogeneous structure, and little is known about how this functional heterogeneity impacts relapse-like behavior for opioids. VP contains subpopulations of neurons with different neurotransmitter profiles and behavioral functions [32–37], and rostrocaudal as well as mediolateral functional heterogeneity are also apparent [38–47]. For example, the rostral portion of VP is critical for cue-induced cocaine seeking, whereas its caudal aspect is instead required for cocaine-primed reinstatement [38]. Caudal VP also contains a ‘hedonic hotspot’ wherein local application of a selective μ opioid receptor agonist (or orexinA peptide [48]) selectively enhances taste pleasure [39, 49].
VP GABAergic (VPGABA) neurons, which span both rostral and caudal VP zones, appear to play a specialized role in reward-related processes, in contrast to intermingled VP glutamate neurons, which instead mediate aversive salience processes [32, 33, 35, 36, 50]. For example, mice find optogenetic stimulation of VPGABA neurons reinforcing, and these neurons show endogenous firing patterns consistent with the encoding of incentive value of rewards and reward-predictive cues [32, 35, 51]. Stimulation of a subset of VPGABA neurons expressing enkephalin also increases reinstatement of cocaine seeking in mice following extinction training (though broadly stimulating VPGABA neurons did not affect reinstatement) [34], and inhibiting VPGABA neurons suppresses context-induced alcohol seeking after extinction training in rats [52]. Though these findings point to an important role for VPGABA neurons in highly motivated and relapse-relevant behaviors, no studies have yet examined their roles in opioid seeking, nor compared their functions in relapse models capturing dissociable addiction-relevant behavioral processes.
Here we address this gap by determining how VPGABA neurons regulate remifentanil intake and seeking using two distinct models of relapse-like behavior, including a newly adapted voluntary abstinence-based reinstatement task. Using DREADDs [53], we found that inhibiting VPGABA neurons decreased opioid relapse after voluntary abstinence, whereas stimulating VPGABA neurons strongly increased opioid seeking regardless of the way in which abstinence was achieved prior to reinstatement. Moreover, chemogenetic effects largely relied on the presence of response-contingent cues, suggesting that VPGABA neurons may play a special role in discrete cue-induced opioid seeking. Consistent with these findings, VP Fos expression correlated with opioid reinstatement behavior in individual animals, but only in its rostral, but not caudal, subregion. Further, we found that neither inhibiting nor stimulating VPGABA neurons influenced unpunished remifentanil self-administration, highlighting a selective role for these neurons in relapse-like drug seeking, rather than in the primary reinforcing effects of remifentanil. Together, these results point to a fundamental and specific role for VPGABA neurons in opioid drug relapse-like behavior in rats, regardless of the behavioral model employed. These results beg the question of whether VP is similarly involved in human drug relapse, and if so, whether such circuits might be a promising future target for clinical treatment of opioid or other addictions [54–56].
Materials and methods
Subjects
GAD1:Cre transgenic rats (n = 32 males, n = 9 females) and wildtype littermates (n = 22 male, n = 12 female) were used in these studies. They were pair-housed in temperature, humidity, and pathogen-controlled cages under a 12:12 hr reverse light/dark cycle, and were provided ad libitum food and water in the homecage throughout all experiments. Experiments were approved by University of California Irvine’s Institutional Animal Care and Use Committee, and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals [57].
Surgery
Procedures for GAD1:Cre-dependent DREADD viral injections in VP were conducted as previously described [58]. Briefly, anesthetized GAD1:Cre rats and wildtype littermates were injected with one of three AAV2 viral constructs obtained from Addgene: hSyn-DIO-hM4D(Gi)-mCherry (n = 11 males, 9 females), hSyn-DIO-hM3D(Gq)-mCherry (n = 36 males, 12 females), or hSyn-DIO-mCherry (n = 7 male, 0 female) (~0.3 μL/hemisphere, titers: ~1.2 × 1013 GC/mL). During the same surgery, rats were implanted with indwelling, back-mounted right jugular vein catheters for chronic drug self-administration as previously described [38, 55, 59, 60].
Drugs
Frozen powder aliquots of clozapine-N-oxide (CNO; NIDA) were diluted in 5% dimethyl sulfoxide (DMSO; Sigma-Aldrich), vortexed for 10 s, then diluted with sterile 0.9% saline to a concentration of 5 mg/mL. CNO was mixed fresh on each test day, and injected at 5 mg/kg (i.p.) 30 min prior to the start of behavioral testing in all experiments. Vehicle solutions were 5% DMSO in saline, injected at 1 mL/kg. Rats were surgically anesthetized with ketamine (56.5 mg/kg) and xylazine (8.7 mg/kg), and given the non-opioid analgesic meloxicam (1 mg/kg). Remifentanil hydrochloride was dissolved in sterile 0.9% saline to a concentration of 38 μg/mL for self-administration.
GroupPunish training
Self-administration phase.
Following recovery from surgery, hM4Di (n = 8 males, 5 females), hM3Dq (n = 13 male, n = 0 female) and control rats (n = 9 males, 8 females) were initially trained in a distinct Context A (peppermint odor, white light, and bare walls) during 2 hr daily sessions. They learned to press an active lever for intravenous infusions of remifentanil (1.9 μg/50 μL/ infusion), a short-acting μ opioid receptor agonist [23, 61], accompanied by a light + tone cue (3.6 s stimulus light + 2.9 kHz tone). Infusions/cues were followed by a 20 s timeout period, signaled by dimming of the houselight, during which lever presses were unreinforced, but recorded. Presses of an inactive lever positioned on the opposite side of the chamber were without consequence. Training in Context A proceeded on the following schedules of reinforcement: 5–6 days of fixed-ratio 1 (FR1), 2 days of variable interval 5 (VI5), 2 days of VI15, and finally 5 days of VI30.
Punishment training.
Next, GroupPunish rats were moved to a distinct Context B (orange odor, red light, and polka dot walls), where active lever presses (on a VI30 schedule) yielded the same dose of remifentanil and the same light + tone cue as delivered in Context A. However, in Context B, infusions were accompanied by a 50% probability of footshock, delivered concurrently with the start of the infusion/cue. All rats were initially given one drug-free punishment training day in Context B (0.30 mA footshock intensity), in order to determine the degree of punishment-induced suppression of self-administration in each individual. An initial cohort (n = 7 hM4Di, n = 5 hM3Dq, n = 4 controls) was then used to examine effects of inhibiting or stimulating VPGABA neurons during punished remifentanil self-administration. This group was administered CNO (5 mg/kg) or vehicle on each subsequent daily punishment training day according to the following protocol: 2 days with 0.30 mA shocks, followed by 2 days each at footshock intensities increasing by 0.15 mA on each step, up to a maximum of 1.65 mA, to suppress pressing. Punishment training ceased in all rats upon reaching voluntary abstinence criterion (< 25 AL presses on 2 consecutive days, days to criterion mean ± SEM: 16.8 ± 0.53). 48 + hours after abstinence criterion was reached in these rats, they were given a final Context B punished self-administration test without CNO/vehicle, to measure maintenance of abstinence in the absence of VP manipulation.
Since no signs of CNO effects were observed on punished drug seeking in this cohort (data not shown), subsequent cohorts of rats (n = 20 males, 7 females) received a modified protocol aimed at more rapidly inducing voluntary abstinence, without daily CNO/vehicle treatment. These rats were trained on the Context B punished self-administration procedure according to the following protocol: 1 day of 0.30 mA shocks, followed by 1 day each at 0.45, 0.60, 0.75, 0.90, and 1.05, 1.20, and 1.35 mA. Rats trained with both protocols reached the same voluntary abstinence criterion (< 25 AL presses on 2 consecutive days) and showed similar levels of pressing by the end of training (average active lever presses on last 2 days in the 2 cohorts: t41 = 0.15, p = 0.88). Both cohorts also showed similar levels of subsequent reinstatement behavior (two-way ANOVA on reinstatement type × cohort; no main effect of cohort: F(1, 121) = 0.28, p = 0.60; or reinstatement type × cohort interaction: F(2, 121) = 1.68, p = 0.19). Therefore, groups were collapsed for subsequent analyses of DREADD effects on reinstatement in GroupPunish.
Reinstatement testing.
After achieving abstinence criterion in Context B, all GroupPunish hM4Di, hM3Dq, and control rats were then administered a series of reinstatement tests to determine how inhibiting or stimulating VPGABA neurons affected reinstatement in Contexts A and B, with or without response-contingent cues (and without further remifentanil or shocks). Counterbalanced vehicle and CNO injections were administered using a within subjects design prior to each reinstatement test with 48 hrs between each test: 1) Context B with response-contingent cues (n = 42) and 2) with no cues (n = 42) and 3) Context A with (n = 43) and 4) with no cues (n = 27). Note that a subset of rats (n = 16) did not undergo the Context A with no cues tests, due to a Spring of 2020 COVID-19 shutdown. Active/inactive lever presses were recorded.
Remifentanil self-administration retraining and testing.
Following reinstatement testing, a subset of GroupPunish rats (n = 5 male, n = 1 female hM4Di, n = 8 male, n = 0 female hM3Dq, n = 6 male, n = 6 female controls) were retrained to self-administer remifentanil and light + tone cue in a distinct chamber (ie, neither Context A nor B) on a VI30 schedule, identical to initial training. Counterbalanced vehicle and CNO tests were administered upon achieving stability criterion (< 25% change in active lever presses on 2 consecutive days), with at least one day of restabilization between tests.
GroupExt training
A separate cohort of hM3Dq rats (n = 8 males, 4 females) and controls (n = 11 males, 4 females) were trained to self-administer remifentanil/cues exactly as was GroupPunish: 14 daily 2 hr sessions up to VI30, occurring in Context A. Next, GroupExt was also moved to Context B, but for this group active lever presses delivered no drug infusions, cues, or shocks (extinction conditions), unlike in GroupPunish where Context B active lever presses yielded all three. Extinction training continued in Context B for GroupExt rats until the extinction criterion was met (< 25 active lever presses on 2 consecutive sessions). After lever pressing was extinguished, GroupExt rats then underwent CNO/vehicle tests on each of the 4 reinstatement types, as described for GroupPunish rats above: 1) Context B with cues (n = 27) and 2) with no cues (n = 27) and 3) Context A with cues (n = 27) and 4) with no cues (n = 27).
hM3Dq-DREADD fos validation
Our prior work validated the function of hM4Di-DREADDs in VPGABA neurons of GAD1:Cre rats [58]. Here, we confirmed the function of hM3Dq-DREADDs in this model, using Fos as a marker of neural activity. To do so, two experimentally-naïve groups were first tested. The first group expressed mCherry in VPGABA neurons (mCherry-only, n = 3), and the second group instead expressed hM3Dq-mCherry in VPGABA neurons (n = 3). Both groups were injected with CNO before returning to the homecage for 2.5 hrs, then were perfused for analysis of Fos in mCherry-expressing VPGABA neurons.
Further, we also asked whether hM3Dq-induced Fos was affected by the behavioral situation the rat was in. In a final 2 hr session following reinstatement testing described above, we stimulated VPGABA neurons of hM3Dq-mCherry GroupExt rats prior to perfusion. These rats were injected with CNO, then 30 min later we noncontingently presented 66 evenly spaced remifentanil-paired cues (n = 3), or no cues (n = 4) over 2 hrs in a novel operant chamber (ie, neither Context A nor B), without levers extended. This number of cues was selected as it was the average number of cues delivered by rats during self-administration training. Rats were perfused immediately after this final cue/no-cue session for analysis of Fos in mCherry-expressing VPGABA neurons.
Experimental procedures
GroupPunish and GroupExt rats were trained on self-administration in Context A, followed by punishment/extinction in Context B, then were tested in a series of reinstatement tests in both contexts, each following counterbalanced vehicle and CNO injections. Some GroupPunish rats (n = 26) were re-trained on remifentanil self-administration following reinstatement to test effects of CNO on self-administration. These rats also underwent a final reinstatement test without CNO, held in Context A or B with cues, to examine behavior-related Fos expression in rostral or caudal VP (neuron type not determined). Some hM3Dq- and mCherry-expressing GroupExt rats (n = 7) and non-behaviorally tested rats (n = 6), following reinstatement tests, were used to validate DREADD stimulation of Fos. For more experimental details about the order of experimental tests in each cohort of animals, see Supplementary Table 1.
Immunofluorescent and immunohistochemical staining
Immunofluorescent visualization of DREADD expression.
To visualize DREADD localization in each behaviorally tested subject, VP sections were stained for substance P, which delineated VP borders from surrounding basal forebrain [37, 62], and mCherry, which labeled DREADD-expressing GABA neurons. Rats were perfused with 0.9% saline and 4% paraformaldehyde, brains were postfixed for 16 hrs, then cryoprotected in 20% sucrose-azide. Brains were sectioned at 40 μm using a cryostat, and 6–8 sections spanning VP’s rostrocaudal axis (from bregma + 0.7 to bregma −0.6) were collected and stained, as described previously [58]. Briefly, sections were first blocked in 3% normal donkey serum (NDS), then incubated overnight in rabbit anti-substance P (ImmunoStar; 1:5000) and mouse anti-mCherry antibodies (Clontech; 1:2000) in PBST-azide with 3% NDS. Finally, sections were incubated for 4 hrs in Alexafluor donkey anti-Rabbit 488 and donkey anti-Mouse 594 (Thermofisher). Sections were mounted, coverslipped with Fluoromount (Thermofisher), and imaged at 5x magnification with a Leica DM4000 with StereoInvestigator software (Microbrightfield). Viral expression sites were mapped in each rat referencing a rat brain atlas [63] and observed VP borders.
Endogenous reinstatement-related VP Fos visualization.
A subset of GroupPunish rats were perfused following a final 2 hr reinstatement test (Context A with cues: n = 9, Context B with cues: n = 8), in the absence of CNO/vehicle injection. To quantify reinstatement-related neural activity in VP cells of any type, we stained a set of slices throughout VP for Fos protein, and co-stained the same samples for substance P to define VP borders on each section. Tissue was blocked in 3% NDS, incubated overnight in rabbit anti-Fos primary antibody (Millipore, 1:10,000), then for 2 hrs in biotinylated donkey anti-rabbit secondary antibody (Jackson Immuno, 1:500), followed by 90 min amplification in avidin-biotin complex (ABC; Vector Lab, 1:500). Sections were then reacted in 3,3′-Diaminobenzidine (DAB) with nickel ammonium sulfate, to reveal a black nuclear stain for Fos protein. After washing, sections were incubated overnight in mouse anti-substance P (Abcam, 1:10,000), then donkey anti-mouse biotinylated secondary antibodies (Jackson Immuno, 1:500), then amplified with ABC. Another DAB reaction without nickel ammonium sulfate was conducted, yielding a light brown product visualizing substance P-immunoreactive processes and neuropil (i.e. VP borders).
Validating hM3Dq-induced Fos in VPGABA neurons.
From separate experimentally-naïve (n = 6) and GroupExt (n = 7) Cre + rats expressing mCherry in VPGABA cells, sections were stained using double DAB immunohistochemistry to visualize neurons expressing Fos (black nuclei) and mCherry (brown soma). Procedures mirrored above, except after the Fos stain, a mouse anti-mCherry primary antibody (Takara Bio, 1:5000) was used instead of the substance P primary antibody to visualize hM3Dq-mCherry or mCherry-expressing cells.
Fos quantification
Endogenous reinstatement-related VP Fos quantification.
To examine reinstatement-related Fos within defined VP borders, stained sections were mounted, coverslipped, and imaged at 10x magnification, and two observers blind to experimental conditions manually counted all Fos + nuclei within the substance-P defined VP borders on 4 sections/rat, using ImageJ. These sections spanned the rostrocaudal extent of VP, from bregma + 0.7 to bregma −0.6. Fos counts from the left and right hemisphere were averaged for each section, and these section averages were averaged to generate a per-rat mean Fos value, which was used for statistical analyses. In addition, sections were divided into rostral and caudal bins (1–3 sections/bin) in accordance with their location relative to bregma (rostral VP > 0 AP relative to bregma, caudal VP ≤ 0 AP relative to bregma). An inter-rater reliability measure showed a strong positive correlation between the two observers’ per-rat average Fos quantification (Pearson’s correlation: r = 0.94, p < 0.0001).
Quantifying hM3Dq-induced Fos in VPGABA neurons.
To examine hM3Dq-induced Fos in VPGABA cells, stained sections were mounted, coverslipped, and imaged at 10x magnification. mCherry + cells, Fos+ cells, and mCherry+/Fos+ cells within VP borders (estimated based on [63]) were counted in ImageJ. Two sections per rat were quantified from near the center of VP virus expression sites, and counts from the left and right hemisphere of each section were averaged. The two section averages were then combined to generate a per-rat mean, which was used for statistical analyses of group effects.
Data analysis
Data were analyzed in Graphpad Prism, and figures were generated in Adobe Illustrator. Repeated measures two-way ANOVAs with lever (inactive, active) and treatment (vehicle, CNO) were conducted for each set of reinstatement and self-administration tests, accompanied by Sidak post hoc tests. One-way or two-way ANOVAs were used to examine differences in lever pressing among vehicle-treated rats during their reinstatement tests, coupled with Sidak post hoc tests. Repeated measures two-way ANOVAs with DREADD type (Gi, Gq, control) and treatment (vehicle, CNO) were used to compare active lever pressing for each reinstatement condition to confirm DREADD-specificity of CNO effects. Three-way repeated measures ANOVAs with treatment (vehicle, CNO), context (Context A, Context B), and cues (cues, no cues) as factors were conducted for each DREADD group. Between subjects three-way ANOVAs with DREADD group (hM4Di, hM3Dq, Control), context (Context A, Context B), and cue (cues, no cues) as factors were performed for vehicle-day reinstatement active lever pressing. A one-way ANOVA was used to compare Fos across reinstatement conditions, coupled with Sidak or Dunnett’s post hoc tests. Paired t-tests were used to compare final-day self-administration behavior to first day punishment behavior. Pearson’s correlations were used to examine the relationship between VP Fos and active lever pressing on a final reinstatement test, as well as inter-rater reliability between blinded observers. Log-rank (Mantel-Cox) test compared the number of days required to reach abstinence criterion for GroupPunish versus GroupExt. One rat in GroupExt and 1 in the substance P/Fos reinstatement experiment were removed from reinstatement and Fos analyses, respectively, as outliers (> 3 standard deviations from the mean). Statistical significance thresholds for all analyses were set at p < 0.05, two-tailed.
Theory
Opioid addiction is a chronically-relapsing disorder, but we have few effective therapies to offer those trying to remain abstinent from opioid drugs. In part, this may be due to 1) a lack of understanding of the neural circuits underlying compulsive use and relapse to opioid seeking, and 2) a failure to capture key aspects of the addiction/relapse cycle in preclinical addiction models. Here, we examine the activity, necessity, and sufficiency of VPGABA neurons for reinstatement of opioid drug seeking using complementary rat behavioral models of relapse, and provide converging evidence for them playing a critical role in opioid relapse.
Results
Training in Context A, and response suppression in Context B via punishment or extinction training
During Context A training, GroupPunish and GroupExt exhibited comparable levels of remifentanil self-administration (Fig. S1A; infusions obtained throughout training: t68 = 1.30, p = 0.20). In GroupPunish, as we previously saw using an analogous cocaine model [55], shifting from unpunished Context A to Context B where 50% of infusions were met with contingent footshock decreased active lever responding (Fig. 1A, last day Context A vs. 1st day Context B: active lever, t42 = 2.61, p = 0.022), and increased inactive lever pressing (t42 = 2.70, p = 0.0098). In GroupExt, shifting from Context A to Context B also decreased active lever responding (Fig. 1C, last day self-administration vs. 1st day extinction active lever: t26 = 3.16, p = 0.004) and increased inactive lever responding (t26 = 5.22, p < 0.0001). Across Context B training, GroupPunish rats suppressed their active lever pressing to criterion in fewer days than GroupExt rats (Fig. S1B, Log-rank Mantel-Cox survival analysis test, χ2 = 18.52, p < 0.0001).
Fig. 1.
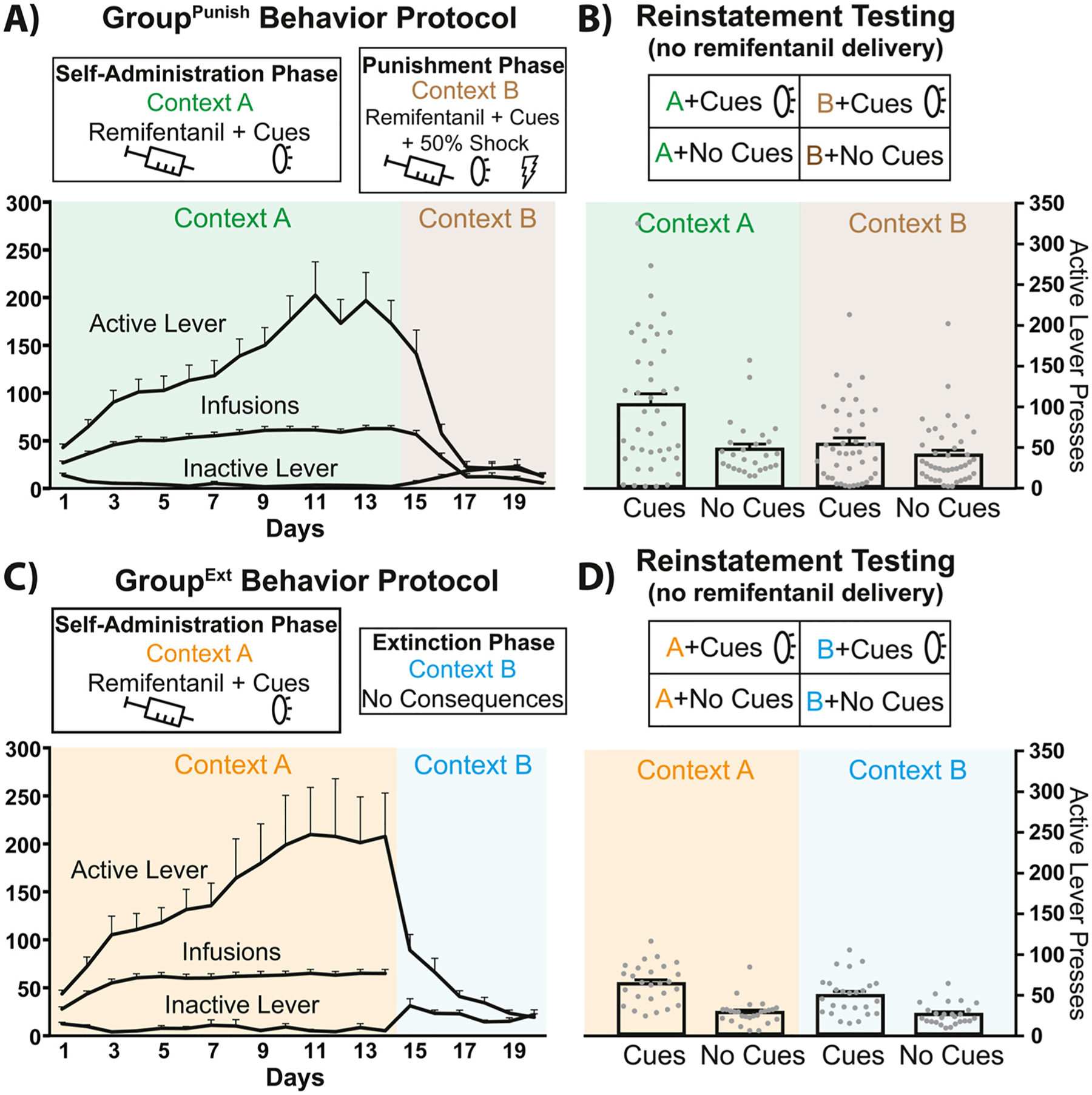
Behavioral testing schematic, training data, and vehicle-day reinstatement following punishment- versus extinction-induced abstinence. A) Schematic of the behavioral training for GroupPunish rats undergoing self-administration in Context A (green shading) and punishment in Context B (brown shading). Infusions and active/inactive lever presses depicted. B) In GroupPunish rats, active lever pressing in Context A (green) and punishment Context B (brown), with or without response-contingent discrete cues is shown for vehicle test days, with accompanying test schematic (top). C) Schematic of the behavioral training for GroupExt rats undergoing self-administration in Context A (orange shading) and extinction in Context B (blue shading). Infusions and active/inactive lever presses depicted. D) In GroupExt rats, active lever pressing in Context A (orange) or extinction Context B (blue), with or without discrete cues is shown for vehicle test days, with associated test schematic (top). All training and testing sessions were 2 hr in duration. Individual rats shown as gray dots. Data presented as mean + SEM.
Cues and contexts gate remifentanil reinstatement in both GroupPunish and GroupExt rats
In GroupPunish rats, opioid reinstatement was impacted by both context and the presence or absence of discrete, response-contingent cues (Fig. 1B, two-way ANOVA (cues and context as factors) on vehicle test day active lever pressing; cues main effect: F(1, 150) = 14.3, p = 0.0002; context main effect: F(1, 150) = 9.23, p = 0.0028; cues × context interaction: F(1, 150) = 5.13, p = 0.025). In Context A, more seeking was seen with cues than without (Sidak post hoc: p = 0.0005), and cues elicited more pressing in Context A than they did in Context B (p = 0.0006). Context A with cue reinstatement was also greater than in Context B without cues (p < 0.0001). pressing was similar in Context A without cues to pressing in Context B, with or without cues (ps > 0.83). In GroupExt rats, opioid reinstatement was also impacted by both context and discrete, response-contingent cues (Fig. 1D, two-way ANVOA (cues and context as factors); cues main effect: F(1, 100) = 55.67, p < 0.0001; context main effect: F(1, 100) = 4.43, p = 0.038; cues × context interaction: F(1, 100) = 2.57, p = 0.11). Pressing was greater in the Context A with cues test than in either context without cues (Sidak post hocs; Context A with cues versus no-cue tests in Context A: p < 0.0001; or Context B: p < 0.0001), and cue-elicited pressing trended toward being greater in Context A than in Context B (p = 0.059). In Context B, pressing was greater with cues than without them (p = 0.0004). Overall, reinstatement in GroupPunish was greater than reinstatement in GroupExt (vehicle day data; two-way ANOVA with group (GroupPunish, GroupExt) and reinstatement condition (Context A with/without cues, Context B with/without cues) as factors: F(1, 250) = 11.44, p = 0.0008). This effect was in part due to high levels of pressing of GroupPunish rats in Context A with cues, as there was greater reinstatement in Context A with cues relative to all other reinstatement conditions in both GroupPunish and GroupExt (Sidak post hocs: ps < 0.016). These results indicate that response-contingent cues reinstate seeking following either punishment or extinction training, but the modulation of this by context may be greater in GroupPunish, relative to GroupExt.
Rostral VP neural activity is positively correlated with cue-induced reinstatement
To determine whether VP Fos (any neuron type) was associated with reinstatement behavior, a subset of GAD1:Cre and wildtype GroupPunish rats, following all 8 reinstatement tests with vehicle and CNO, were sacrificed following a final reinstatement test in Context A with cues, Context B with cues, or directly from their homecage. Greater Fos expression in all VP neurons was found in both Context A- and Context B-tested rats, relative to homecage-tested controls (Fig. 2A, one-way ANOVA, F(2, 21) = 12.25, p = 0.0003; Dunnett’s post hoc: Context A with cues vs. homecage, p = 0.005; Context B with cues vs. homecage, p = 0.0002). No difference in VP Fos expression was detected between Context A with cues and Context B with cues (Sidak post hoc: p = 0.43). Fos in rostral (Fig. 2B), but not caudal (Fig. 2C) VP correlated with total active lever presses during the final reinstatement test (r = 0.62, p = 0.014), similar to our prior report showing that rostral VP Fos is associated with cue-induced cocaine seeking [38].
Fig. 2.
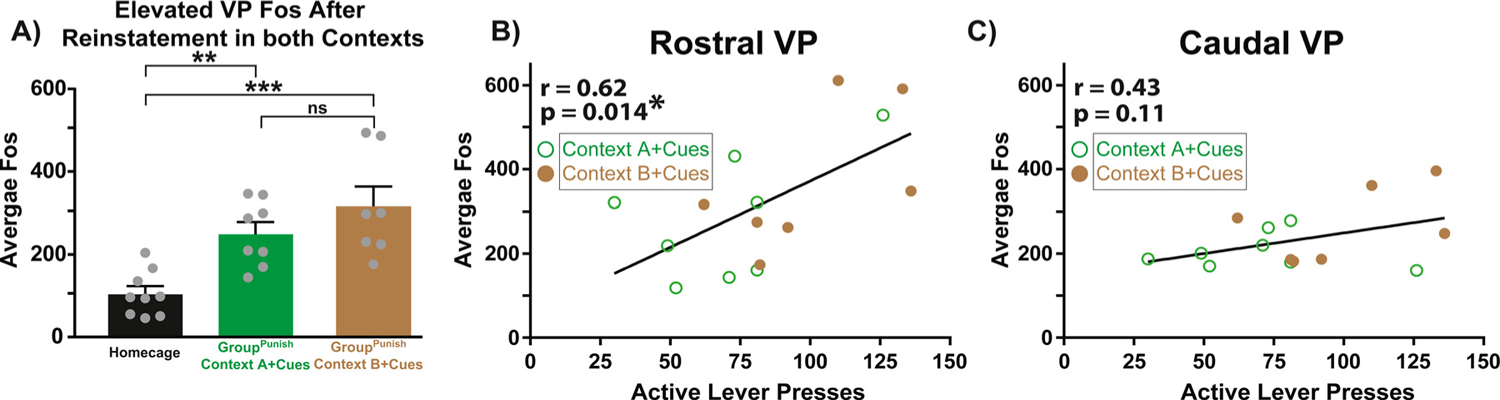
Rostral, but not caudal, VP Fos correlates with remifentanil seeking. A) Elevated Fos in VP neurons in Context A with cues (green bar) and Context B with cues (brown bar), relative to homecage control (gray bar). No difference in VP Fos was detected between Context A with cues and Context B with cues. Individual rats shown as gray dots. Data presented as mean + SEM. B) In GroupPunish rats, Fos in rostral VP (anterior of bregma) positively correlates with cue-induced opioid reinstatement (active lever presses). Green circles represent rats tested with cues in Context A, brown dots represent those tested with cues in Context B. C) Fos in caudal VP (posterior of bregma) was uncorrelated with opioid reinstatement. Pearson correlation: p* < 0.05. One-way ANOVA, Dunnett’s post hoc: p** < 0.01, p*** < 0.0001.
hM4Di- and hM3Dq-DREADD expression in VPGABA neurons
GAD1:Cre rats expressing DREADDs with at least 50% within VP borders (defined by substance P) were included for analyses, for a total of 13 GAD1:Cre hM4Di-expressing (hM4Di) rats (Fig. 3A–B, GroupPunish: n = 8 males, 5 females) and 25 GAD1:Cre hM3Dq-expressing (hM3Dq) rats (Fig, 3C-D, D, GroupPunish: n = 13 males, 0 females; GroupExt: n = 8 males, 4 females). Control rats were designated as those with DREADD expression outside of VP (n = 2), mCherry expression (n = 8), or Cre-rats with no expression (n = 10), which were combined for analyses.
Fig. 3.
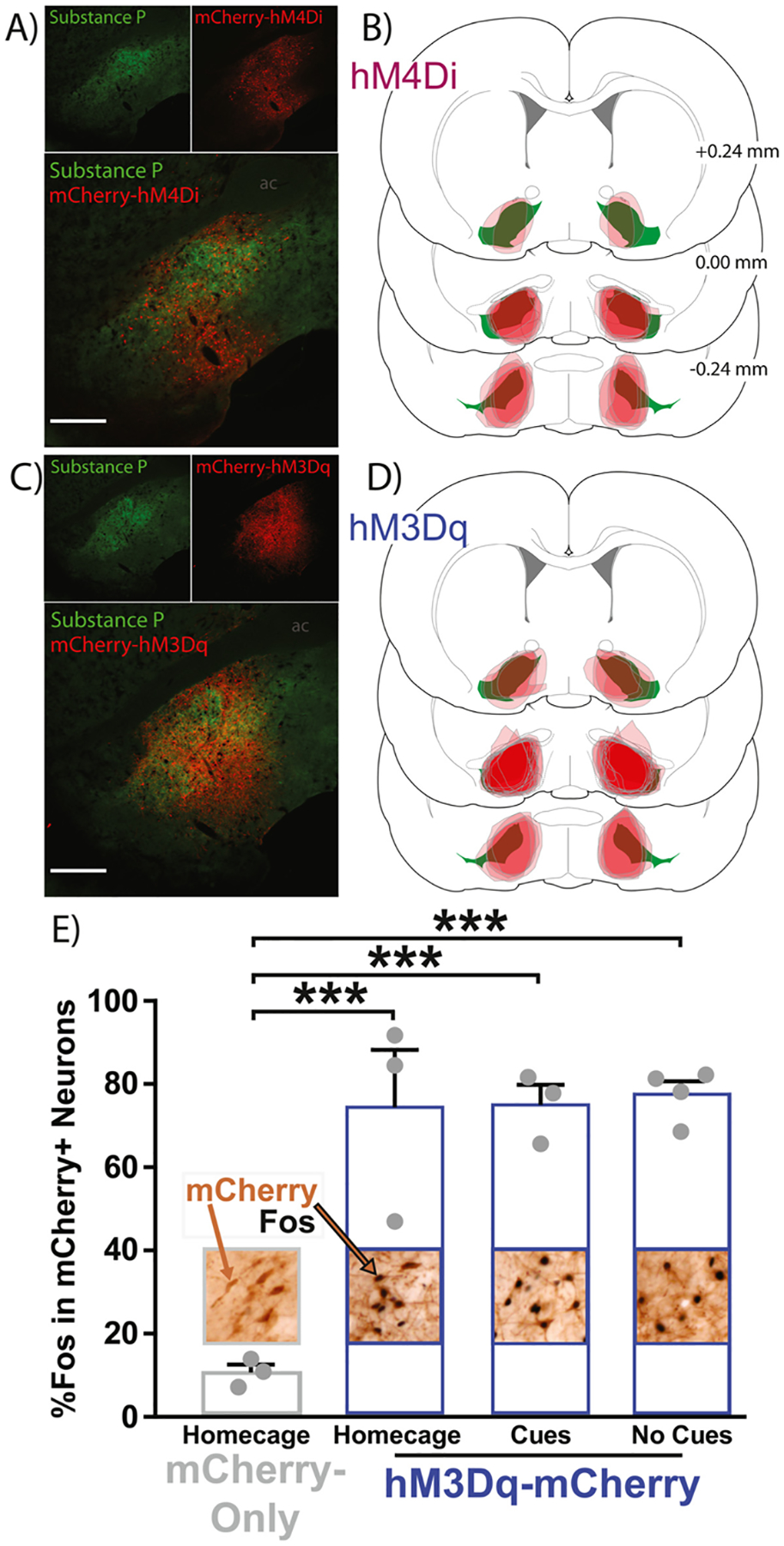
VPGABA DREADD localization and hM3Dq validation. A) Expression of hM4Di-mCherry (red) localized largely within VP borders defined by substance P (green). B) Coronal sections depicting the center of hM4Di-mCherry expression (red) for each rat along VP’s rostrocaudal axis relative to bregma (substance P-defined VP borders = green). C) Expression of hM3Dq-mCherry (red) is similarly localized within VP borders (green). D) Coronal sections similarly depicting the center of hM3Dq-mCherry expression (red) for each rat is shown. E) CNO treatment in hM3Dq-mCherry rats tested in the homecage exhibited greater Fos in mCherry + neurons (2nd bar, blue), than in homecage mCherry-only rats treated with CNO (1st bar, gray). CNOtreated hM3Dq-mCherry rats exposed to remifentanil-paired cues (3rd bar, blue) or no cues (4th bar, blue) had more Fos + mCherry neurons homecage rats (1st bar, gray). However, the hM3Dq stimulation of Fos was no different in the presence or absence of cues. Images above/embedded within bars depict 10x images of immunohistochemical staining of mCherry (brown) within VP borders, and Fos + nuclei (black). Example mCherry-only and mCherry + Fos double-labeled neuron indicated with brown and brown/black arrows, respectively. Individual rat data shown as gray dots on top of bars. One-way ANOVA, Dunnett’s post hoc: p*** < 0.001. Data presented as mean + SEM.
CNO increases Fos immunoreactivity in hM3Dq-expressing neurons
CNO treatment induced more Fos in hM3Dq-expressing neurons, relative to mCherry-only neurons (Fig. 3E, one-way ANOVA: F(3, 9) = 20.56, p = 0.0002). Equivalent Fos induction was seen regardless of the behavioral circumstance in which CNO was administered, with similar homecage mCherry-relative increases in hM3Dq rats tested in homecage (Dunnett’s post hoc, p = 0.0005), or in a novel operant chamber with (p = 0.0004) or without (p = 0.0002) cues. hM3Dq rats tested in home- or test-cages did not differ in Fos expression (Sidak post hoc: ps > 0.99). These results collectively show that 1) hM3Dq stimulation augments neural activity as expected and 2) hM3Dq stimulation enhanced neural activity similarly regardless of the behavioral context in which the stimulation occurred.
Inhibiting VPGABA neurons suppresses remifentanil reinstatement after punishment
In GroupPunish rats expressing hM4Di DREADDs, a lever (active, inactive) × treatment (vehicle, CNO) ANOVA revealed a significant main effect of lever across all conditions (p < 0.01). Active lever presses in Context A with cues were suppressed by CNO treatment in hM4Di rats (Fig. 4A, treatment × lever interaction: F(1, 24) = 6.53, p = 0.017; active lever Sidak post hoc: p = 0.0047), but this was not the case in Context A without cues (Fig. 4D, treatment: F(1, 10) = 0.43, p = 0.52; treatment × lever interaction: F(1, 10) = 3.38, p = 0.096), showing that inhibiting VPGABA neurons suppressed seeking in Context A only in the presence of discrete cues. Moreover, vehicle day reinstatement was statistically comparable across DREADD groups (DREADD main effect: F(2,11) = 2.31, p = 0.10; DREADD × cue × context interaction: F(2,11) = 0.23, p = 0.80). CNO treatment in hM4Di rats trended towards reducing opioid seeking in Context B with cues (Fig. 4G, treatment × lever interaction: F(1, 24) = 3.98, p = 0.058), but no main effect of treatment, or treatment × lever interaction was detected in Context B without cues (Fig. 4J, treatment: F(1, 24) = 2.79, p = 0.11; treatment × lever: F(1, 24) = 0.12, p = 0.73). A three-way RM ANOVA (treatment × context × cues) revealed no significant interaction for hM4Di rats (F(1,5) = 0.25, p = 0.64).
Fig. 4.

Following punishment, inhibiting or stimulating VPGABA neurons bidirectionally controls remifentanil seeking. A–C) In Context A with cues, CNO treatment A) decreased opioid seeking in hM4Di rats (purple bar), B) increased seeking in hM3Dq rats (blue bar), and C) was without effect in control rats (light gray bar), relative to vehicle treatment (black bars). d–F) In Context A with no cues, CNO treatment was without effect on opioid seeking in D) hM4Di rats (purple bar), E) hM3Dq rats (blue bar), and F) control rats (light gray bar), relative to vehicle treatment day (black bars). G–I) In Context B with cues, CNO treatment G) was without effect on opioid seeking in hM4Di rats (purple bar), H) increased opioid seeking in hM3Dq rats (blue bar), and F) did not impact opioid seeking in control rats (light gray bar), relative to vehicle treatment day (black bars). J-L) In Context B with no cues, CNO treatment was without effect on opioid seeking in J) hM4Di rats (purple bar), K) hM3Dq rats (blue bar), and L) control rats (light gray bar), relative to vehicle treatment (black bars). Dark gray overlaid bars with white outline represent inactive lever presses, and light gray lines depict individual rats’ active lever pressing on each session. Repeated measures two-way ANOVA, Sidak post hoc: p** < 0.01, p*** < 0.001. Data presented as mean + SEM.
Stimulating VPGABA neurons augments remifentanil reinstatement after punishment
In GroupPunish rats expressing hM3Dq DREADDs in VPGABA neurons, CNO strongly increased opioid seeking, and appeared to do so in a cue-dependent manner. Specifically, CNO augmented active lever pressing in both Context A with cues and Context B with cues (Fig. 4B, Context A with cues treatment × lever interaction: F(1, 24) = 8.78, p = 0.0068, active lever Sidak post hoc: p = 0.0001; Fig. 4H, Context B with cues treatment × lever interaction: F(1, 24) = 9.79, p = 0.0046, active lever Sidak post hoc: p = 0.0001). In contrast, CNO in hM3Dq rats failed to augment seeking in Context A in the absence of cues (Fig. 4E, Context A with no cues treatment × lever interaction: F(1, 14) = 0.22, p = 0.64; Context A with no cues treatment: F(1, 14) = 2.08, p = 0.17). CNO in hM3Dq rats subtly increased pressing on both the active and inactive lever in Context B with no cues, as indicated by a main effect of treatment accompanied by a non-significant treatment × lever interaction (Fig. 4K, Context B with no cues treatment: F(1, 24) = 4.77, p = 0.039; Context B with no cues treatment × lever interaction: F(1, 24) = 0.92, p = 0.35; active lever Sidak post hoc: p = 0.071; inactive lever: p = 0.63). A three-way RM ANOVA (treatment × context × cues) revealed no significant interaction in hM3Dq rats (F(1,7) = 0.0001, p = 0.98). For active lever timecourse details for each reinstatement/DREADD condition in GroupPunish rats, see Supplemental Fig. 2.
Stimulating VPGABA neurons augments remifentanil seeking after extinction
In GroupExt rats with hM3Dq DREADDs, CNO treatment augmented seeking in the presence of cues, irrespective of whether rats were in Context A or B (Fig. 5A, treatment main effect Context A with cues: F(1, 20) = 4.91, p = 0.038, active lever Sidak post hoc: p = 0.037; Fig. 5E, treatment main effect Context B with cues: F(1, 20) = 9.86, p = 0.0052, active lever Sidak post hoc: p = 0.033). In the absence of cues, CNO augmented opioid reinstatement only in Context A, but not in Context B (Fig. 5C, treatment main effect Context A with no cues: F(1, 20) = 8.84, p = 0.0075, active lever Sidak post hoc: p = 0.011; Fig. 5G, treatment main effect Context B with no cues: F(1, 20) = 2.10, p = 0.16; treatment × lever interaction: F(1, 20) = 0.19, p = 0.67). A three-way RM ANOVA (treatment × context × cues) revealed no significant interaction in hM3Dq rats (F(1,10) = 0.04, p = 0.84). Overall, we find that stimulating VPGABA neurons in GroupPunish or GroupExt rats augments seeking in either Context in the presence of cues, but only increases non-cued seeking in Context A in GroupExt but not in GroupPunish.
Fig. 5.
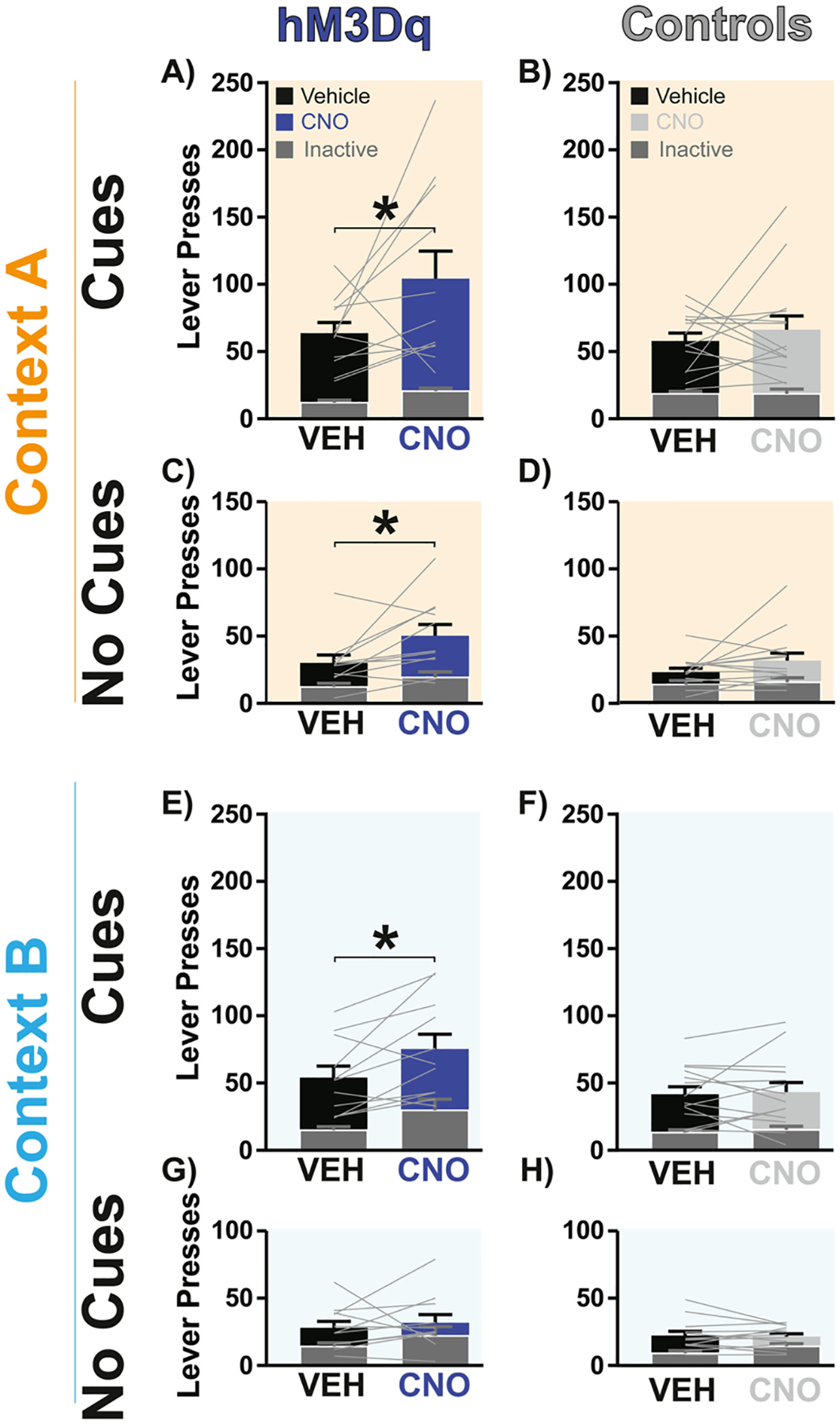
Following extinction, stimulating VPGABA neurons augments reinstatement in a cue- and context-dependent manner. A-B) CNO treatment (blue bar) in hM3Dq rats augmented opioid seeking in Context A with cues relative to vehicle (black bar), but no effect was detected in controls (light gray bar versus black bar). C-D) CNO treatment (blue bar) in hM3Dq rats increased opioid seeking in Context A with no cues relative to vehicle, but no effect was seen in controls (light gray bar versus black bar). E-F) CNO (blue bar) in hM3Dq rats increased opioid seeking in Context B with cues, relative to vehicle treatment (black bar), but no effect was observed in controls (light gray bar versus black bar). G-H) CNO treatment in hM3Dq rats (blue bar) or controls (light gray bar) was without effect on opioid reinstatement in Context B with no cues, relative to vehicle treatment (black bars). Dark gray overlaid bars with white outline represent inactive lever presses, and light gray lines depict individual rats’ active lever pressing. p* < 0.05. Data presented as mean + SEM.
Neither inhibiting nor stimulating VPGABA neuron alters opioid self-administration
Finally, we asked whether VPGABA neuron manipulations influence unpunished opioid self-administration in a subset of GroupPunish rats, retrained to self-administer after reinstatement testing. Stable self-administration was unaffected by CNO treatment in hM4Di rats (Fig. 6A, treatment: F(1, 10) = 2.14, p = 0.17; treatment × lever interaction: F(1, 10) = 2.14, p = 0.17) or hM3Dq rats (Fig. 6C, treatment: F(1, 10) = 1.63, p = 0.23; treatment × lever interaction: F(1, 10) = 1.63, p = 0.23). The number of infusions obtained was similarly unaffected by VPGABA neuron manipulations (Fig. 6B, hM4Di: t5 = 1.89, p = 0.12; Fig. 6D, hM3Dq: t5 = 1.98, p = 0.10).
Fig. 6.

No impact of inhibiting or stimulating VPGABA neurons on remifentanil self-administration. Relative to vehicle day performance in the same rats (black bars), CNO treatment failed to alter A) active lever presses or B) infusions obtained during unpunished opioid self-administration in hM4Di rats (purple bars). C,D) Analogous self-administration data is shown for hM3Dq rats (blue bars), and E,F) control rats (gray bars). Gray lines represent individual rats’ behavioral output. Data presented as mean + SEM.
No effect of CNO on behaviors in control rats
In GroupPunish control rats, CNO did not influence opioid reinstatement in Context A with cues (Fig. 4C) or without them (Fig. 4F), or in Context B with (Fig. 4I) or without cues (Fig. 4L, treatment: Fs ⟨0.86, ps⟩ 0.35; treatment × lever interaction: Fs ⟨ 1.28, ps ⟩ 0.26). Similarly, in GroupExt control rats, CNO did not impact reinstatement after extinction in Context A with cues (Fig. 5B) or with no cues (Fig. 5D), or Context B with (Fig. 5F) or with no cues (Fig. 5H, treatment: Fs ⟨ 3.56, ps ⟩ 0.06; treatment × lever interaction: Fs ⟨ 2.61, ps ⟩ 0.11). CNO (versus vehicle) showed no main effect (GroupPunish: F(1,11) = 0.47, p = 0.51; GroupExt: F(1,14) = 1.98, p = 0.18) or interactions with cue or context variables (three-way RM ANOVA (treatment × context × cues): GroupPunish: F(1,11) = 0.01, p = 0.92; GroupExt: F(1,14) = 0.002, p = 0.97). Likewise, CNO was also without effect on remifentanil self-administration in controls (Fig. 6E, treatment: F(1, 20) = 0.98, p = 0.34; treatment × lever interaction: F(1, 20) = 1.07, p = 0.31; Fig. 6F, control infusions: t10 = 0.082, p = 0.94). Specificity of CNO effects were confirmed with two-way ANOVAs examining DREADD group × treatment effects on active lever pressing in CNO-impacted reinstatement conditions. For GroupPunish rats, on reinstatement tests for which CNO had an effect, we found specificity of CNO effects (DREADD × treatment interactions: Fs ⟩ 12.63, ps ⟨0.0001). For GroupExt rats, given that there were only hM4Di and control groups, DREADD × treatment interactions were non-significant (Fs ⟨4.09, ps ⟩ 0.054).
Discussion
Using chemogenetic inhibition/stimulation and Fos expression analyses, we found that VPGABA neurons play a key role in opioid relapse-like behavior. Following remifentanil self-administration and subsequent abstinence from drug taking, chemogenetically inhibiting VPGABA neurons suppressed, and stimulation enhanced opioid reinstatement—especially when it was driven by discrete, response-contingent drug cues. VPGABA’s role was apparent across multiple reinstatement models, and it was specific to reinstatement, in that the same chemogenetic manipulations did not affect remifentanil’s primary reinforcing properties. We also validated hM3Dq DREADDs as being capable of Fos-activating GABA neurons in GAD1:Cre transgenic rats, and determined that VPGABA neurons were equivalently stimulated by DREADDs in the presence or absence of drug-associated cues. This is despite the fact that the presence of cues during such stimulation was generally required for increased drug-seeking behavior to occur. Finally, we found that endogenous neural activity (Fos) in rostral, but not caudal, VP cells correlated with reinstatement behavior. These experiments thus show a specific role for VPGABA neurons in opioid relapse-like behaviors, regardless of the preclinical model employed—potentially positioning VP as a future target for intervention in this chronic, relapsing disorder.
In hopes of better modeling the circumstances of drug addiction, preclinical models have emerged in which drug taking is coupled with adverse consequences which cause rats to decide to quit using [6, 54, 64, 65]-similar to the self-imposed abstinence present in most humans attempting to control their drug intake. We and others have suggested that through such efforts to better model human addiction and relapse-like behaviors in rats we may gain new insights into the neural circuit dynamics most likely engaged in addicted humans. Here, we build on prior work to establish a model of remifentanil cue- and context-induced relapse after punishment-induced abstinence, adapting those previously used with other drugs of abuse [55, 66–69]. Using the short-acting but strongly reinforcing opioid drug remifentanil, we built on the work of Panlilio and colleagues who previously established that footshock punishment suppresses remifentanil self-administration, and that remifentanil seeking can be subsequently reinstated [11, 22]. Here, we expand on these models by incorporating an explicit contextual element to the reinstatement tests (with or without discrete drug-paired cues), allowing us to interrogate the facilitatory and suppressive effects of these learned stimuli on drug seeking. We note that unlike extinction- or forced abstinence-based reinstatement models, voluntary abstinence models may mimic the conflicted motivational processes that often arise in addicted people attempting to control their drug use due to mounting life consequences. We hope that developing this approach in rats could ultimately lead to deeper understanding of neural circuits that are engaged when humans decide to try to quit using.
Discrete cues occurring in conjunction with drug use (e.g., paraphernalia), and diffuse contextual elements (eg, location of prior drug use) serve as powerful triggers that can ultimately lead an abstinent person to relapse. The ability of discrete cues and contexts to elicit drug seeking appears to depend on overlapping yet distinct neural circuits [54, 70, 71], some of which involve VP or its close neural connections [38, 52, 72–77]. Therefore, we examined VPGABA involvement in reinstatement elicited by both discrete cues and contexts in our behavioral relapse models. After punishment-induced abstinence, inhibiting VPGABA neurons only reduced remifentanil seeking in the “safe” Context A in the presence of cues—the condition in which reinstatement was highest. In contrast, we found that inhibiting VPGABA neurons did not affect seeking in the punishment-associated Context B in the presence or absence of cues, potentially in part due to a floor effect resulting from low responding in this “dangerous” context. These results are reminiscent of our prior report with cocaine showing that chemogenetic inhibition of VP neurons suppressed cue-induced drug seeking in a safe Context A, but not in a dangerous Context B, using an analogous voluntary abstinence-based reinstatement model [55]. Here, we also examined effects of stimulating VPGABA neurons on post-punishment opioid seeking, which we found to robustly augment cue-induced remifentanil seeking in both Context A and Context B. In the absence of cues, however, stimulating VPGABA neurons exhibited no effect in either Context A or B. It appears, then, that response-contingent cues are required to reveal the motivation-enhancing effects observed with hM3Dq stimulation after punishment-induced abstinence. Overall, these data suggest cue- and context-dependent roles for VPGABA in reinstatement following voluntary abstinence.
Though we found cue- and context-dependent effects of manipulating VPGABA neurons on opioid seeking following punishment-induced abstinence, the way in which abstinence is achieved in preclinical models determines the neural circuits recruited during reinstatement [12, 54, 65, 78]. Therefore, we asked whether stimulating VPGABA neurons would have similar effects on cue or context-induced reinstatement using an analogous extinction-based abstinence reinstatement model. We found that VPGABA neuron stimulation in extinguished rats similarly augmented cue-induced remifentanil seeking in both Context A and B. However, unlike in punishment-trained rats, VPGABA neuron stimulation in extinguished rats augmented seeking in Context A in the absence of cues, not just in their presence. This could suggest that VPGABA roles in context-induced reinstatement may differ based on the affective associations imbued in these contexts (i.e. fear of shock versus extinction-related disengagement), or contrast effects between the always safe Context A with the extinction- or punishment-paired Context B. Alternatively, differences between the models in the number of training days required for extinction- versus punishment-induced abstinence (Fig. S1), or other methodological differences between the procedures could have contributed to this distinction. Future work ought also to explore how punishment learning in Context A (rather than a distinct Context B) might impact neural circuit recruitment and reinstatement. Regardless, these findings of a relatively pervasive, necessary and sufficient role for VPGABA neurons in cue-induced reinstatement contrast with other limbic nodes like the basolateral amygdala or dorsal striatum, since inactivating these nodes differentially affects reinstatement behavior depending on the way in which abstinence was achieved [12, 78]. Overall, these results suggest that VPGABA neurons are involved in cue-induced drug seeking across rat relapse models, suggesting they might also play an analogous role in human opioid relapse.
Prior work from us and others suggests nuanced roles for VP and its neuronal subpopulations in drug seeking, as well as motivated behavior more generally [37, 40, 58, 79–81]. In particular, recent reports support a role for phenotypically-defined VP cellular subpopulations in relapse to drug seeking across drugs of abuse and relapse models [34, 38, 55, 72, 82–85]. For example, VP dopamine D3-receptor expressing populations and their outputs to lateral habenula are critical for cue-induced cocaine seeking [82]. VPGABA and parvalbumin-expressing VP neurons are also recruited by alcohol-associated contextual cues, and chemogenetic inhibition of these subpopulations suppressed context-induced relapse to alcohol seeking [52]. Stimulation of a subset of enkephalin-expressing VPGABA neurons enhanced cue-induced cocaine reinstatement, whereas stimulating VP glutamate neurons instead suppressed cocaine reinstatement [34]. However, although broad stimulation of VPGABA neurons induced reinstatement to cocaine seeking in extinguished mice, it failed to augment cue-induced reinstatement [34]. These findings are collectively in accordance with the idea that VP glutamate neurons constrain reward seeking, and have opposite motivational roles to VPGABA neurons, which are instead involved in appetitive processes [32, 33, 35, 58]. Our results further demonstrate the critical role of VPGABA neurons in opioid seeking, especially when triggered by drug-paired cues.
Consistent with prior reports examining cocaine seeking, we identified that rostral, but not caudal, VP neural activity (Fos) was positively correlated with cue-induced drug seeking [38], and that VP neural activity was elevated following reinstatement testing in both punishment-associated Context B or reward-associated Context A55. Our Fos results demonstrate that rostral, not caudal VP is activated when rats undergo cue-induced reinstatement of remifentanil seeking, and that rostral VP Fos scales with the intensity of drug seeking across individual animals. Several groups have also shown a functional gradient along VP’s rostrocaudal axis [41, 43–47]. For example, caudal VP contains a ‘hedonic hotspot’ in which locally applied orexinA or μ opioid receptor agonists enhance hedonic orofacial ‘liking’ reactions to sweet liquid rewards [39, 48]. Though our Fos analysis (Fig. 2) was not restricted to VPGABA cells, the majority of Fos-positive cells were likely GABAergic, since VP consists of mostly GABAergic neurons across its rostrocaudal axis [32, 37, 86]. We also note that our DREADD manipulations were targeted in central VP, and therefore spanned both rostral and caudal VP zones. Future work should further dissect anatomical, cellular, and molecular profiles spanning rostrocaudal VP zones to determine the specific roles of neuronal populations within these subregions responsible for this apparent anterior-posterior functional gradient.
We show that Gq-DREADDs robustly stimulate Fos in VPGABA neurons, as seen in other neural populations [38, 60, 87–90], and here we further asked whether the behavioral situation impacts hM3Dq-induced Fos, as it does for hM3Dq-induced behavior. Specifically, we reasoned that since hM3Dq-stimulated reinstatement was most robust in the presence of response-contingent cues, the presentation of these cues might further augment hM3Dq-simulated Fos levels, relative to rats exposed to no cues, or tested outside a drug-seeking context (homecage). However, the presence or absence of discrete, passively administered cues made no difference—Fos levels in mCherry-hM3Dq VP neurons following CNO administration were similar regardless of whether testing occurred in the presence of drug cues. This indicates that hM3Dq DREADD stimulation enhances activity of VPGABA neurons regardless of behavioral situation, though the same stimulation only caused consistent increases in drug seeking the presence of cues. This likely implies that VP activation either results in drug seeking or does not depending on the cue-elicited activity state of the wider motivation circuits within which VP interacts. Perhaps this is not surprising conceptually—enhanced activity of the VPGABA projection neurons cannot inhibit cue-evoked activity in target regions (e.g. VTA) if cues are not present to cause activity capable of being inhibited. In theory, this could be a useful feature of manipulating such inhibitory circuits to treat psychiatric disorders, as the behavioral/cognitive effects of activating GABAergic projections may only become apparent in the presence of symptom-related circumstances related to abnormal neuronal hyperactivity in downstream regions.
These studies have some limitations that should be considered, and explicitly followed up in future studies. For example, both male and female rats were used in these studies, but in some cases unequal numbers of each sex were present, precluding our ability to examine sex differences in most cases. This said, in our prior work we detected no sex differences in effects of chemogenetic manipulations of VP neurons, or VPGABA neurons [55, 58], though sex-dependent VP effects are still likely [91–93] and worthy of additional study. CNO doses equivalent or higher than that used here (5 mg/kg) have no discriminable effects on reinstatement or self-administration behaviors in our hands [38, 55, 58, 60]. Likewise, no observable effects of CNO were seen in non-DREADD expressing control rats here—though off target effects of the compound have been reported and should always be controlled for [94, 95]. We did not test here effects of VPGABA neuron inhibition on post-extinction reinstatement, only stimulation. Several prior reports have shown VP is essential for post-extinction reinstatement [30, 38, 52, 72, 96, 97], but the pattern of effects in our specific context/cue model are unknown. Though our prior results have shown that chemogenetic VPGABA neuron manipulations do not affect locomotor activity per se [55, 58], we did not explicitly control for such locomotor effects in the current study. Finally, it is important to note that DREADD stimulation of neurons is unlikely to recapitulate natural firing patterns generated endogenously by VP circuits, and VP firing dynamics should be further studied using complementary methods.
Our results establish that VPGABA neurons regulate reinstatement across preclinical opioid relapse models, adding to the growing evidence for a key role of VP within motivation circuits [24, 25, 37, 98–104]. Though such evidence implies that targeting VP circuits might be a useful strategy for helping humans struggling to control their drug use, many questions remain. How does molecular heterogeneity within neurotransmitter-defined VP circuits influence motivated behavior? Is it possible to modulate the activity of VP to influence maladaptive drug seeking without impairing healthy desires? How is VP’s efferent and afferent connectivity involved in different types of motivated behavior? Further investigating these questions may yield fruitful insights not only about VP’s role in addiction, but also fundamental ways in which motivational systems interact with cognitive and memory systems more generally.
Supplementary Material
Funding
This work was supported by grants from NIDA R00-DA035251, P50-DA044118, F31-DA048578, NINDS F99-NS120641, the State of California Tobacco-Related Disease Research Program T31IR1767, and a Hellman Foundation Fellowship.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.addicn.2022.100026.
References
- [1].O’Brien CP, Childress AR, McLellan AT, Ehrman R, Classical conditioning in drug-dependent humans a, Ann. N. Y. Acad. Sci 654 (1) (1992) 400–415. [DOI] [PubMed] [Google Scholar]
- [2].Sinha R, Li CSR, Imaging stress-and cue-induced drug and alcohol craving: association with relapse and clinical implications, Drug Alcohol Rev 26 (1) (2007) 25–31. [DOI] [PubMed] [Google Scholar]
- [3].American Psychiatric ADiagnostic and Statistical Manual of Mental Disorders (DSM-5®), American Psychiatric Pub, 2013. [Google Scholar]
- [4].Serre F, Fatseas M, Swendsen J, Auriacombe M, Ecological momentary assessment in the investigation of craving and substance use in daily life: a systematic review, Drug Alcohol Depend 148 (2015) 1–20. [DOI] [PubMed] [Google Scholar]
- [5].Shaham Y, Shalev U, Lu L, De Wit H, Stewart J, The reinstatement model of drug relapse: history, methodology and major findings, Psychopharmacology (Berl.) 168 (1–2) (2003) 3–20. [DOI] [PubMed] [Google Scholar]
- [6].Venniro M, Banks ML, Heilig M, Epstein DH, Shaham Y, Improving translation of animal models of addiction and relapse by reverse translation, Nat. Rev. Neurosci (2020) 1–19. [DOI] [PubMed] [Google Scholar]
- [7].Ahmed SH, Lenoir M, Guillem K, Neurobiology of addiction versus drug use driven by lack of choice, Curr. Opin. Neurobiol 23 (4) (2013) 581–587. [DOI] [PubMed] [Google Scholar]
- [8].Vanderschuren LJMJ, Minnaard AM, Smeets JAS, Lesscher HMB, Punishment models of addictive behavior, Curr. Opin. Behav. Sci 13 (2017) 77–84. [Google Scholar]
- [9].Lüscher C, Robbins TW, Everitt BJ, The transition to compulsion in addiction, Nat. Rev. Neurosci (2020) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Belin D, Belin-Rauscent A, Everitt BJ, Dalley JW, In search of predictive endophenotypes in addiction: insights from preclinical research, Genes Brain Behav 15 (1) (2016) 74–88. [DOI] [PubMed] [Google Scholar]
- [11].Panlilio LV, Thorndike EB, Schindler CW, Lorazepam reinstates punishment-suppressed remifentanil self-administration in rats, Psychopharmacology (Berl.) 179 (2) (2005) 374–382. [DOI] [PubMed] [Google Scholar]
- [12].Pelloux Y, Minier-Toribio A, Hoots JK, Bossert JM, Shaham Y, Opposite effects of basolateral amygdala inactivation on context-induced relapse to cocaine seeking after extinction versus punishment, J. Neurosci 38 (1) (2018) 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Epstein DH, Preston KL, Stewart J, Shaham Y, Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure, Psychopharmacology (Berl.) 189 (1) (2006) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y, Neurobiology of the incubation of drug craving, Trends Neurosci 34 (8) (2011) 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y, Relapse to opioid seeking in rat models: behavior, pharmacology and circuits, Neuropsychopharmacology 44 (3) (2019) 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kruyer A, Chioma VC, Kalivas PW, The opioid-addicted tetrapartite synapse, Biol. Psychiatry 87 (1) (2020) 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shaham Y, Erb S, Stewart J, Stress-induced relapse to heroin and cocaine seeking in rats: a review, Brain Res. Rev 33 (1) (2000) 13–33. [DOI] [PubMed] [Google Scholar]
- [18].Fredriksson I, Venniro M, Reiner DJ, Chow JJ, Bossert JM, Shaham Y, Animal models of drug relapse and craving after voluntary abstinence: a review, Pharmacol. Rev 73 (3) (2021) 1050–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grimm JW, Hope BT, Wise RA, Shaham Y, Incubation of cocaine craving after withdrawal, Nature 412 (6843) (2001) 141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fuchs RA, See RE, Basolateral amygdala inactivation abolishes conditioned stimulus-and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats, Psychopharmacology (Berl.) 160 (4) (2002) 425–433. [DOI] [PubMed] [Google Scholar]
- [21].De Wit H, Stewart J, Reinstatement of cocaine-reinforced responding in the rat, Psychopharmacology (Berl.) 75 (2) (1981) 134–143. [DOI] [PubMed] [Google Scholar]
- [22].Panlilio LV, Thorndike EB, Schindler CW, Reinstatement of punishment-suppressed opioid self-administration in rats: an alternative model of relapse to drug abuse, Psychopharmacology (Berl.) 168 (1–2) (2003) 229–235. [DOI] [PubMed] [Google Scholar]
- [23].Burkle H, Dunbar S, Van Aken H, Remifentanil: a novel, short-acting, mu-opioid, Anesthesia Analgesia 83 (3) (1996) 646–651. [DOI] [PubMed] [Google Scholar]
- [24].Napier TC, Mitrovic I, Opioid modulation of ventral pallidal inputs, Ann. N. Y. Acad. Sci 877 (1) (1999) 176–201. [DOI] [PubMed] [Google Scholar]
- [25].Smith KS, Tindell AJ, Aldridge JW, Berridge KC, Ventral pallidum roles in reward and motivation, Behav. Brain Res 196 (2) (2009) 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Austin MC, Kalivas PW, Enkephalinergic and GABAergic modulation of motor activity in the ventral pallidum, J. Pharmacol. Exp. Therapeut 252 (3) (1990) 1370–1377. [PubMed] [Google Scholar]
- [27].Caillé S, Parsons LH, Intravenous heroin self-administration decreases GABA efflux in the ventral pallidum: an in vivo microdialysis study in rats, Eur. J. Neurosci 20 (2) (2004) 593–596. [DOI] [PubMed] [Google Scholar]
- [28].Caillé S, Parsons LH, Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway, Neuropsychopharmacology 31 (4) (2006) 804. [DOI] [PubMed] [Google Scholar]
- [29].Hubner CB, Koob GF, The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat, Brain Res 508 (1) (1990) 20–29. [DOI] [PubMed] [Google Scholar]
- [30].Rogers JL, Ghee S, See RE, The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse, Neuroscience 151 (2) (2008) 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mohammadkhani A, Fragale JE, Pantazis CB, Bowrey HE, James MH, Aston-Jones G, Orexin-1 receptor signaling in ventral pallidum regulates motivation for the opioid remifentanil, J. Neurosci 39 (49) (2019) 9831–9840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Faget L, Zell V, Souter E, et al. , Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum, Nat. Commun 9 (1) (2018) 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tooley J, Marconi L, Alipio JB, et al. , Glutamatergic ventral pallidal neurons modulate activity of the habenula–tegmental circuitry and constrain reward seeking, Biol. Psychiatry 83 (12) (2018) 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Heinsbroek JA, Bobadilla A-C, Dereschewitz E, et al. , Opposing regulation of cocaine seeking by glutamate and GABA neurons in the ventral pallidum, Cell Rep 30 (6) (2020) 2018–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Stephenson-Jones M, Bravo-Rivera C, Ahrens S, et al. , Opposing contributions of GABAergic and glutamatergic ventral pallidal neurons to motivational behaviors, Neuron 105 (5) (2020) 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Geisler S, Derst C, Veh RW, Zahm DS, Glutamatergic afferents of the ventral tegmental area in the rat, J. Neurosci 27 (21) (2007) 5730–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Root DH, Melendez RI, Zaborszky L, Napier TC, The ventral pallidum: subregion-specific functional anatomy and roles in motivated behaviors, Prog. Neurobiol 130 (2015) 29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mahler SV, Vazey EM, Beckley JT, et al. , Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking, Nat. Neurosci 17 (4) (2014) 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Smith KS, Berridge KC, Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum, J. Neurosci 27 (7) (2007) 1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Root DH, Ma S, Barker DJ, et al. , Differential roles of ventral pallidum subregions during cocaine self-administration behaviors, J. Compar. Neurol 521 (3) (2013) 558–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Churchill L, Kalivas PW, A topographically organized gamma-aminobutyric acid projection from the ventral pallidum to the nucleus accumbens in the rat, J. Compar. Neurol 345 (4) (1994) 579–595. [DOI] [PubMed] [Google Scholar]
- [42].Zahm DS, Williams E, Wohltmann C, Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain, J. Compar. Neurol 364 (2) (1996) 340–362. [DOI] [PubMed] [Google Scholar]
- [43].Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C, Specificity in the projection patterns of accumbal core and shell in the rat, Neuroscience 41 (1) (1991) 89–125. [DOI] [PubMed] [Google Scholar]
- [44].Calder AJ, Beaver JD, Davis MH, Van Ditzhuijzen J, Keane J, Lawrence AD, Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods, Eur. J. Neurosci 25 (11) (2007) 3422–3428. [DOI] [PubMed] [Google Scholar]
- [45].Johnson PI, Stellar JR, Paul AD, Regional reward differences within the ventral pallidum are revealed by microinjections of a mu opiate receptor agonist, Neuropharmacology 32 (12) (1993) 1305–1314. [DOI] [PubMed] [Google Scholar]
- [46].Kupchik YM, Kalivas PW, The rostral subcommissural ventral pallidum is a mix of ventral pallidal neurons and neurons from adjacent areas: an electrophysiological study, Brain Struct. Function 218 (6) (2013) 1487–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Panagis G, Miliaressis E, Anagnostakis Y, Spyraki C, Ventral pallidum self-stimulation: a moveable electrode mapping study, Behav. Brain Res 68 (2) (1995) 165–172. [DOI] [PubMed] [Google Scholar]
- [48].Ho C-Y, Berridge KC, An orexin hotspot in ventral pallidum amplifies hedonic ‘liking’for sweetness, Neuropsychopharmacology 38 (9) (2013) 1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Smith KS, Berridge KC, The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking ” and food intake, J. Neurosci 25 (38) (2005) 8637–8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang F, Zhang J, Yuan Y, et al. , Salience processing by glutamatergic neurons in the ventral pallidum, Sci. Bull 65 (5) (2020) 389–401. [DOI] [PubMed] [Google Scholar]
- [51].Zhu C, Yao Y, Xiong Y, et al. , Somatostatin neurons in the basal forebrain promote high-calorie food intake, Cell Rep 20 (1) (2017) 112–123. [DOI] [PubMed] [Google Scholar]
- [52].Prasad AA, Xie C, Chaichim C, et al. , Complementary roles for ventral pallidum cell types and their projections in relapse, J. Neurosci 40 (4) (2020) 880–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL, Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand, Proc. Natl. Acad. Sci 104 (12) (2007) 5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Farrell MR, Schoch H, Mahler SV, Modeling cocaine relapse in rodents: behavioral considerations and circuit mechanisms, Prog. Neuropsychopharmacol. Biol. Psychiatry 87 (Pt A) (2018) 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Farrell MR, Ruiz CM, Castillo E, et al. , Ventral pallidum is essential for cocaine relapse after voluntary abstinence in rats, Neuropsychopharmacology (2019) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McGovern DJ, Root DH, Ventral pallidum: a promising target for addiction intervention, Neuropsychopharmacology 44 (13) (2019) 2151–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].National Research CGuide For the Care and Use of Laboratory Animals, National Academies Press, 2010. [Google Scholar]
- [58].Farrell MR, Esteban JSD, Faget L, Floresco SB, Hnasko TS, Mahler SV, Ventral pallidum GABA neurons mediate motivation underlying risky choice, J. Neurosci 41 (20) (2021) 4500–4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mahler SV, S Aston-Jones G, Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats, J. Neurosci 32 (38) (2012) 13309–13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mahler SV, Brodnik ZD, Cox BM, et al. , Chemogenetic manipulations of ventral tegmental area dopamine neurons reveal multifaceted roles in cocaine abuse, J. Neurosci 39 (3) (2019) 503–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Egan TD, Remifentanil pharmacokinetics and pharmacodynamics, Clin. Pharmacokinet 29 (2) (1995) 80–94. [DOI] [PubMed] [Google Scholar]
- [62].Zahm DS, Heimer L, Ventral striatopallidal parts of the basal ganglia in the rat: I. Neurochemical compartmentation as reflected by the distributions of neurotensin and substance P immunoreactivity, J. Compar. Neurol 272 (4) (1988) 516–535. [DOI] [PubMed] [Google Scholar]
- [63].Paxinos G, Watson C, The Rat Brain in Stereotaxic coordinates: Hard Cover Edition, Elsevier, 2006. [Google Scholar]
- [64].Marchant NJ, Li X, Shaham Y, Recent developments in animal models of drug relapse, Curr. Opinion Neurobiol 23 (4) (2013) 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Marchant NJ, Campbell EJ, Pelloux Y, Bossert JM, Shaham Y, Context-induced relapse after extinction versus punishment: similarities and differences, Psychopharmacology (Berl.) 236 (1) (2019) 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pelloux Y, Hoots JK, Cifani C, et al. , Context-induced relapse to cocaine seeking after punishment-imposed abstinence is associated with activation of cortical and subcortical brain regions, Addict. Biol 23 (2) (2018) 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Marchant NJ, Khuc TN, Pickens CL, Bonci A, Shaham Y, Context-induced relapse to alcohol seeking after punishment in a rat model, Biol. Psychiatry 73 (3) (2013) 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Krasnova IN, Marchant NJ, Ladenheim B, et al. , Incubation of methamphetamine and palatable food craving after punishment-induced abstinence, Neuropsychopharmacology 39 (8) (2014) 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fredriksson I, Applebey SV, Minier-Toribio A, Shekara A, Bossert JM, Shaham Y, Effect of the dopamine stabilizer (-)-OSU6162 on potentiated incubation of opioid craving after electric barrier-induced voluntary abstinence, Neuropsychopharmacology 45 (5) (2020) 770–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Crombag HS, Bossert JM, Koya E, Shaham Y, Context-induced relapse to drug seeking: a review, Philos. Trans. R. Soc. B 363 (1507) (2008) 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bossert JM, Marchant NJ, Calu DJ, Shaham Y, The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research, Psychopharmacology (Berl.) 229 (3) (2013) 453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Prasad AA, McNally GP, Ventral pallidum output pathways in context-induced reinstatement of alcohol seeking, J. Neurosci 36 (46) (2016) 11716–11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Stefanik MT, Kupchik YM, Brown RM, Kalivas PW, Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking, J. Neurosci 33 (34) (2013) 13654–13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Perry CJ, McNally GP, A role for the ventral pallidum in context-induced and primed reinstatement of alcohol seeking, Eur. J. Neurosci 38 (5) (2013) 2762–2773. [DOI] [PubMed] [Google Scholar]
- [75].Kupchik Y, Prasad AA, Ventral pallidum cellular and pathway specificity in drug seeking, Neurosci. Biobehav. Rev (2021). [DOI] [PubMed] [Google Scholar]
- [76].McFarland K, Lapish CC, Kalivas PW, Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior, J. Neurosci 23 (8) (2003) 3531–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Fuchs RA, Ramirez DR, Bell GH, Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats, Psychopharmacology (Berl.) 200 (4) (2008) 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fuchs RA, Branham RK, See RE, Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate–putamen, J. Neurosci 26 (13) (2006) 3584–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Richard JM, Ambroggi F, Janak PH, Fields HL, Ventral pallidum neurons encode incentive value and promote cue-elicited instrumental actions, Neuron 90 (6) (2016) 1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ottenheimer DJ, Bari BA, Sutlief E, et al. , A quantitative reward prediction error signal in the ventral pallidum, Nat. Neurosci 23 (10) (2020) 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Leung BK, Balleine BW, Ventral pallidal projections to mediodorsal thalamus and ventral tegmental area play distinct roles in outcome-specific Pavlovian-instrumental transfer, J. Neurosci 35 (12) (2015) 4953–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pribiag H, Shin S, Wang EH−J, et al. , Ventral pallidum DRD3 potentiates a pallido-habenular circuit driving accumbal dopamine release and cocaine seeking, Neuron (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pardo-Garcia TR, Garcia-Keller C, Penaloza T, et al. , Ventral pallidum is the primary target for accumbens D1 projections driving cocaine seeking, J. Neurosci 39 (11) (2019) 2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Heinsbroek JA, Neuhofer DN, Griffin WC, et al. , Loss of plasticity in the D2-accumbens pallidal pathway promotes cocaine seeking, J. Neurosci 37 (4) (2017) 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Prasad AA, McNally GP, The ventral pallidum and relapse to alcohol seeking, Br. J. Pharmacol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bernat N, Campbell R, Nam H, et al. Distinct properties in ventral pallidum projection neuron subtypes 2021.
- [87].Haaranen M, Scuppa G, Tambalo S, et al. , Anterior insula stimulation suppresses appetitive behavior while inducing forebrain activation in alcohol-preferring rats, Transl. Psychiatry 10 (1) (2020) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sharma PK, Wells L, Rizzo G, et al. , DREADD activation of pedunculopontine cholinergic neurons reverses motor deficits and restores striatal dopamine signaling in parkinsonian rats, Neurotherapeutics (2020) 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yoshimura M, Nishimura K, Nishimura H, et al. , Activation of endogenous arginine vasopressin neurons inhibit food intake: by using a novel transgenic rat line with DREADDs system, Sci. Rep 7 (1) (2017) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Nation HL, Nicoleau M, Kinsman BJ, Browning KN, Stocker SD, DREADD-induced activation of subfornical organ neurons stimulates thirst and salt appetite, J. Neurophysiol 115 (6) (2016) 3123–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].DiBenedictis BT, Cheung HK, Nussbaum ER, Veenema AH, Involvement of ventral pallidal vasopressin in the sex-specific regulation of sociosexual motivation in rats, Psychoneuroendocrinology 111 (2020) 104462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lim MM, Murphy AZ, Young LJ, Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster), J. Compar. Neurol 468 (4) (2004) 555–570. [DOI] [PubMed] [Google Scholar]
- [93].Lee JDA, Reppucci CJ, Bowden SM, Huez EDM, Bredewold R, Veenema AH, Structural and functional sex differences in the ventral pallidal vasopressin system are associated with the sex-specific regulation of juvenile social play behavior in rats, bioRxiv (2021). [Google Scholar]
- [94].Gomez JL, Bonaventura J, Lesniak W, et al. , Chemogenetics revealed: DREADD occupancy and activation via converted clozapine, Science 357 (6350) (2017) 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mahler SV, Aston-Jones G, CNO Evil? Considerations for the use of DREADDs in behavioral neuroscience, Neuropsychopharmacology 43 (5) (2018) 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].McFarland K, Kalivas PW, The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior, J. Neurosci 21 (21) (2001) 8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].McFarland K, Davidge SB, Lapish CC, Kalivas PW, Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior, J. Neurosci 24 (7) (2004) 1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Tachibana Y, Hikosaka O, The primate ventral pallidum encodes expected reward value and regulates motor action, Neuron 76 (4) (2012) 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ottenheimer D, Richard JM, Janak PH, Ventral pallidum encodes relative reward value earlier and more robustly than nucleus accumbens, Nat Commun 9 (1) (2018) 4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Creed M, Ntamati NR, Chandra R, Lobo MK, Lüscher C, Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum, Neuron 92 (1) (2016) 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kaplan A, Mizrahi-Kliger AD, Israel Z, Adler A, Bergman H, Dissociable roles of ventral pallidum neurons in the basal ganglia reinforcement learning network, Nat. Neurosci 23 (4) (2020) 556–564. [DOI] [PubMed] [Google Scholar]
- [102].Levi LA, Inbar K, Nachshon N, et al. , Projection-specific potentiation of ventral pallidal glutamatergic outputs after abstinence from cocaine, J. Neurosci (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Inbar K, Levi LA, Bernat N, Odesser T, Inbar D, Kupchik YM, Cocaine dysregulates dynorphin modulation of inhibitory neurotransmission in the ventral pallidum in a cell-type-specific manner, J. Neurosci 40 (6) (2020) 1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Floresco SB, West AR, Ash B, Moore H, Grace AA, Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission, Nat. Neurosci 6 (9) (2003) 968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


