FIGURE 1. Integrin domains, conformational change, and effect of drugs on the αIIbβ3 headpiece.
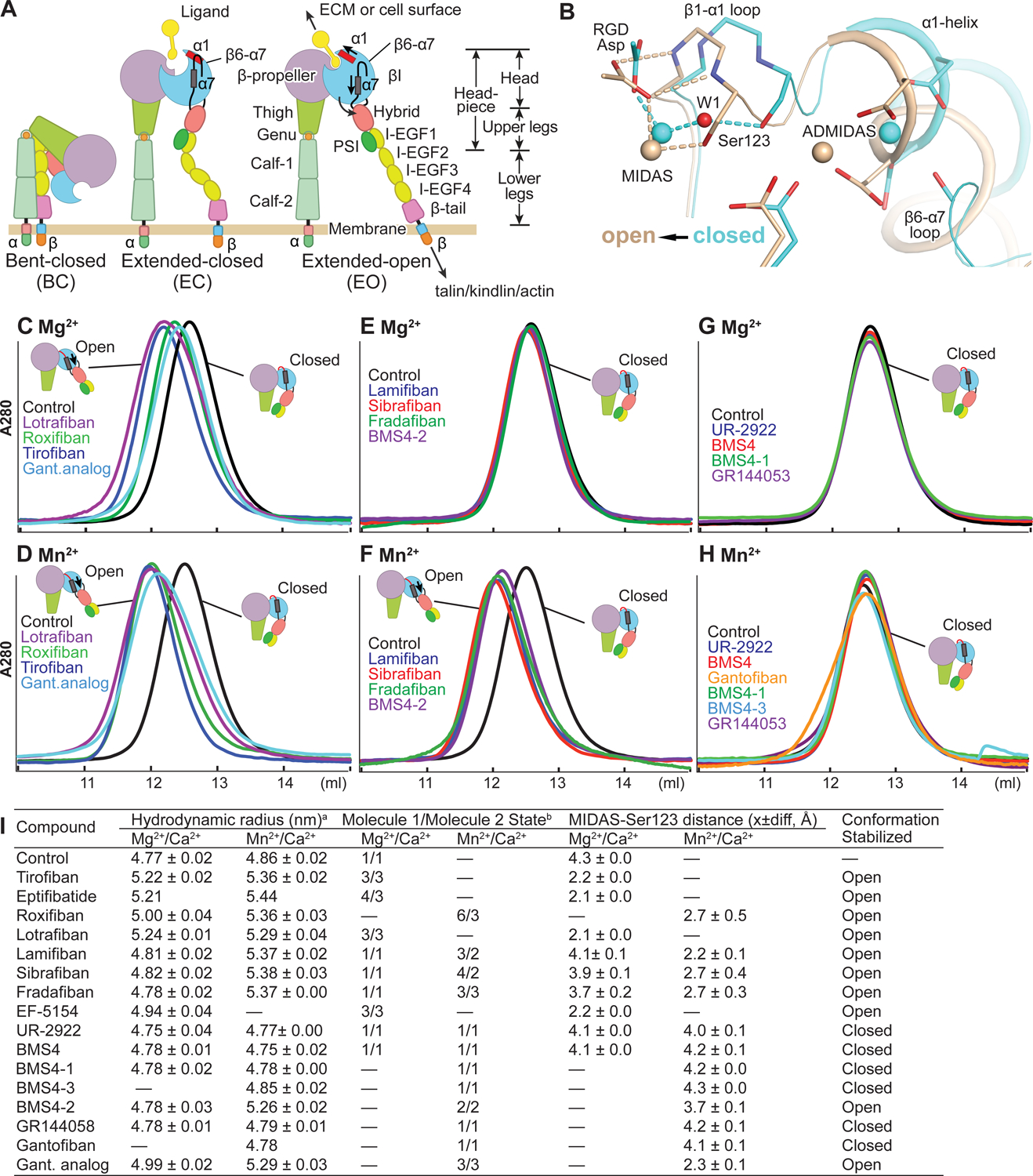
(A) Integrin domain organization and conformational states. In integrin headpiece opening, which increases affinity for biological ligands, pistoning of the α7-helix (purple bar) of the βI domain is linked to α1-helix pistoning (red bar), swing-out of the hybrid domain (curved arrow) and (B) rearrangement of loops at the ligand-binding site. (B) Shows αIIbβ3 bound to RGD in the closed (cyan carbons and metals) and open (wheat carbons and metals) conformations from PDB codes 3ZDY and 3ZE2 chains C+D+J, respectively. Loops and helices are thin and thicker tubes. Key metal coordinating sidechains, backbone and the RGD Asp sidechain are shown in stick. Oxygens are red and nitrogens blue. Metal ions and water 1 are shown as spheres. (C-H) Overlaid Superdex 200 chromatograms in absence (control, black) and presence of 10 μM drug in running buffer with curves in same color as the name of the drug. Running buffer contained 1 mM Mg2+, 1 mM Ca2+ or 2 mM Mn2+ 0.2 mM Ca2+ as indicated. Open and closed headpieces are shown in cartoon in insets (I) Summary of effects of drugs in gel filtration and when soaked into closed αIIbβ3 headpiece crystals. aHydrodynamic radii from Superdex 200 gel filtration. Control and tirofiban measurements are mean and s.d. of ≥ 3 experiments; other measurements are mean and difference from mean of two experiments, except for eptifibatide and gantofiban which were run once. bConformational states (Zhu et al., 2013). MIDAS metal ion - Ser-123 sidechain oxygen atom distances are shown as mean and difference for the two headpiece molecules in the asymmetric unit.
