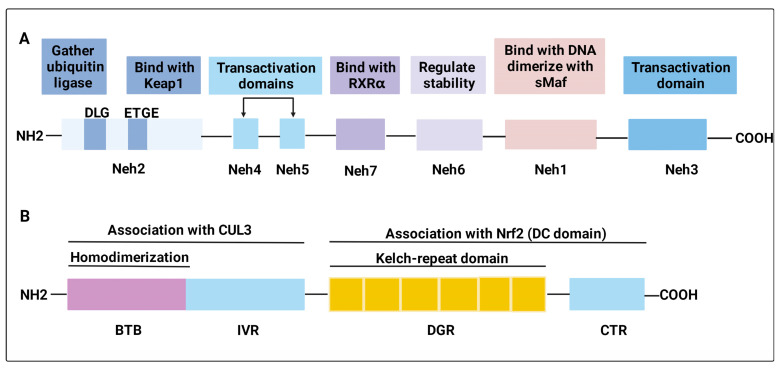
Figure 2.
Structures of Nrf2 and Keap1. (A) The Nrf2 protein comprises seven Neh domains, known as Neh1–Neh7. The Neh1 domain is responsible for DNA binding and dimerization with the sMaf proteins; the Neh2 domain mediates the interaction with Keap1 through the ETGE motifs and gathers a ubiquitin ligase to the fusion protein through the DLG element; the Neh3, Neh4, and Neh5 domains are transactivation domains; the Neh6 domain regulates Nrf2 stability; and the Neh7 domain binds with RXRα to weaken the cytoprotective effect of Nrf2. (B) Keap1 possesses three functional domains. The BTB domain mediates Keap1 homodimerization and associates with Cul3; the DC directly associates with Nrf2 Neh2 domain; IVR domain contains critical cysteine residues and connects the BTB domain with DC domain.