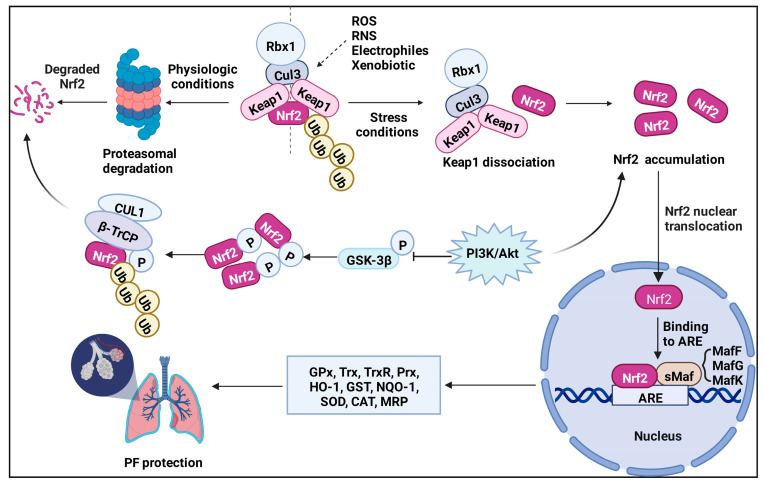
Figure 3.
Mechanism of Nrf2 activation. Under normal physiological conditions, Nrf2 is sequestered in the cytoplasm by its physical interaction with Keap1 and is degraded by the ubiquitin-proteasome in the Keap1-dependent manner or the Keap1-independent manner (GSK-3β pathway). In the stress conditions, Keap1 is inactivated while Nrf2 is stabilized. The stabilized Nrf2 performs nuclear translocation and heterodimerizes with sMaf to activate target genes for cell protection through ARE. Nrf2 target genes include GPx, Trx, TrxR, Prx, HO−1, GST, NQO1, SOD, and CAT which defend lungs from further damages. Furthermore, Nrf2 also can be directly phosphorylated by GSK-3β, enabling its recognition by β-TRCP, which later marks Nrf2 for ubiquitination and degradation through the proteasome. However, PI3K/AKT signaling pathway can suppress GSK-3β by AKT-mediated phosphorylation and causing Nrf2 accumulation in the cytoplasm. The accumulation of Nrf2 further activates transcription of a number of cytoprotective genes and protection against pulmonary fibrosis.