Background
The use of race in medicine implies that we are physiologically different based on our outward, physical characteristics. However, race is not based in genetics, nor in physiology, but is entirely a social construct based on characteristics, physical locations, and behavioral patterns.1
We have incorporated race into multiple clinical equations despite unclear evidence for doing so. We also recognize that the effects of racism and other social determinants of health, rather than race itself, are responsible for disparities in health outcomes.
We highlight in this paper the use of race-based glomerular filtration rate (GFR). It has been suggested that the current race-based algorithm incorporating GFR is delaying diagnosis and treatment of worsening chronic kidney disease.2
Why GFR Matters for Kidney Disease
GFR tells us how well the kidneys are working, and is used to estimate kidney function and thus chronic kidney disease (CKD). While estimated GFR (eGFR) can decline with age, CKD risk factors include diabetes and hypertension. GFR is not only important for evaluation of chronic kidney disease but is also considered while ordering medications that could be renally eliminated.3
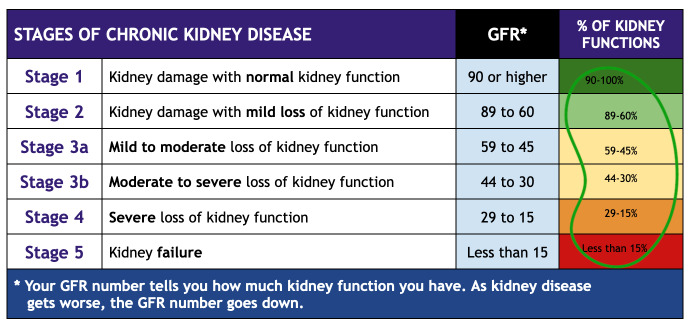
Since measured GFR is expensive and lengthy, we currently use creatinine as a filtration marker to estimate GFR using the CKD-EPI and Modification of Diet in Renal Disease (MDRD) equations.4 Both equations include adjustments based on age, sex, and race. An eGFR of less than 90 mL/min/1.73 m2 is categorized into five stages of chronic kidney disease with initiation of dialysis at stage 5 (Figure 1).
Figure 1.
Stages of Chronic Kidney Disease
These eGFR equations are based on small and flawed studies published between the 1970s and 1990s. The MDRD equation was developed in 1999 using data from 1628 patients; of those, 197 Black men and women were included in the cohort.5 During the study, there was an incidental finding that Black men and women had higher creatinine excretion rates compared to their White counterparts. This happened to coincide with a report from the preceding year from the National Health and Nutrition Examination Survey, which also suggested the same but did not have any objective data on GFR and prevalence of CKD.5 The coincidental findings led researchers to conclude that Black race was an independent predictor of GFR, leading to the addition of the race coefficient to the equation without accounting for social factors or other independent predictors of CKD including hypertension and diabetes.
Adjusting for Black race using the MDRD and CKD-EPI equations accounts for an approximately 18% and 16% increase in eGFR, respectively. It was assumed that Black people have a higher average muscle mass (possibly due to diet or genetic differences), which has never been proven by any reputable research. While dietary differences can be cultural, they are also influenced by socioeconomic status, so this is likely a confounder and poor surrogate marker of directly influencing creatinine. It is an inappropriate generalization to assume Black people have more muscle mass. One cannot even measure muscle mass on living humans; it can only be measured on a cadaver.
Public Health & Policy Considerations
Physicians are failing to diagnose early stages of CKD in the Black population, which leads to a delay in secondary prevention.2 The leading causes of chronic kidney failure include diabetes and hypertension, both which can be controlled with the appropriate treatment regimens to prevent further progression of CKD. While early-stage kidney disease can be asymptomatic, it can progress to later stages without appropriate treatment and lead to fatigue, swelling in the extremities, muscle cramps and oliguria.6 Diagnosing CKD at earlier stages is critical so physicians can evaluate underlying causes and control risk factors.
Despite the Black population constituting only 13% of the overall US population, Black patients account for more than 35% of all patients in the United States receiving dialysis for kidney failure.7 The disproportionate number of Black patients receiving dialysis further supports that the Black population has worse outcomes for chronic kidney disease, yet we continue to calculate and assign a higher GFR value with the current equations.
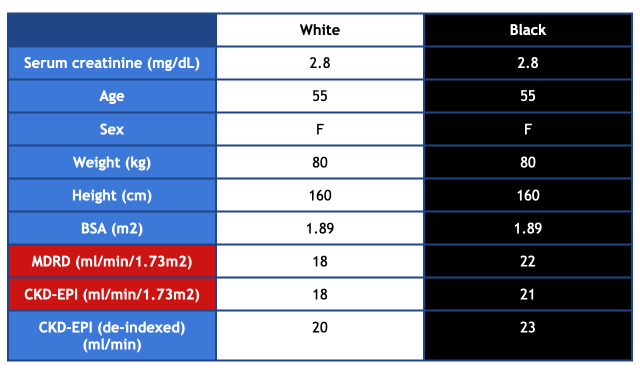
For example, a 55-year-old woman with a serum creatinine of 2.8 comes to the clinic for evaluation. If she is a White woman, she would be referred for a transplant due to an eGFR of 20 mL/min/1.73 m2 using the CKD-EPI equation. A Black woman with the same weight, height, body surface area and creatinine would not be referred because her GFR using CKD-EPI would be calculated as 23 mL/min/1.73 m2 (Figure 2). In nephrology, an eGFR 20 mL/min/1.73 m2 or less is the threshold for referral in the United States. Kidney transplant is the optimal treatment with ESRD, yet Black patients are less likely to be referred for transplant, and once on the list, wait longer than their White counterparts. A recent study at Brigham and Women’s hospital showed that removing the race coefficient would reclassify 3.1% of Black patients from eGFR > 20 mL/min/1.73 m2 to eGFR ≤ 20 mL/min/1.73 m2, making them eligible for a transplant referral.2
Figure 2.
Comparison of MDRD and CKD-EPI Values for White and Black Women.
Addressing such disparities in health is not only important from a social justice and equity standpoint but also for improving the nation’s overall health and economic prosperity. A national study updated in 2018 by the W.K. Kellogg Foundation showed that there is a potential economic gain of $135 billion per year if racial disparities in health care were eliminated.8 This includes $93 billion in excess medical care costs and $42 billion in untapped productivity.8 As our population becomes more diverse, it is important to address these disparities.
Racism & Health
Aside from the obvious delays in care for CKD, there are multiple other issues with using the race coefficient. For example, one obvious issue lies in the following question – how do providers categorize individuals with a heterogenous heritage? Currently, there is no guidance in this area. In the United States, whether you have one-fourth, one-eighth, or less Black ancestry, you are considered Black. During the Jim Crow segregation era, the South had the “one drop rule,” which meant a single drop of “Black blood” makes a person Black.9 Similarly, medicine is making arbitrary distinctions that are not based in science. It is left to the provider’s discretion to decide which race category to use based on his or her understanding of a patient’s race or the patient chooses which one they self-identify with the most. This, again, subverts the concept that race is a legitimate scientific variable. As Yearby wrote, “The genome between socially constructed racial groups is 99.5%-99.9% identical; the 0.1%-0.5% variation between any two unrelated individuals is greatest between individuals in the same racial group; and there are no identifiable racial genomic clusters.”10 In our view, this suggests that structural racism continues to affect modern medicine. This will become a more pressing issue as there was a 276% increase in individuals identifying as mixed race in recent years, which is predicted to increase.11
Future Directions: Towards a Race-Neutral Consideration of Kidney Disease
What happens if we eliminate the race coefficient? A recent cross-sectional study showed that removing race as a factor in recommending care resulted in one third of Black patients reclassified to a more severe stage of CKD.12 This initiative will improve the quality of life for one third of the population with CKD and prevent early progression to ESRD. Physicians can order early referrals and transplant evaluations. While some may argue that ending the race adjustment can lead to overdiagnosis and overtreatment, as medical professionals we should be favoring practices that alleviate health inequities over those that will exacerbate them.
The National Kidney Foundation and the American Society of Nephrology (NFK-ASN) created a task force that recently completed a 10-month review process with social and scientific evidence, testimonies from patients, providers and trainees including 97 experts representing 21 US states and seven other countries.13 They examined in detail twenty-six eGFR approaches with the intent to eliminates bias and shortened the initial twenty-six approaches to six by eliminating those that would be difficult to implement or have significant barriers in terms of standardizing in labs. Creatinine, for example, is widely used and standardized while cystatin C is only available in some labs and would be difficult to immediately implement.
Of the twelve approaches that did not include race as a variable, seven were not developed in a population that included Black individuals. This led to a final five approaches for further review to find an accurate, non-racially based, and feasible cost-effective measurement for GFR. The committee then divided the potential clinical consequences into general medical care and nephrology care with general medical care being medication initiation and dosing. Based on patient testimonies and research, it was determined that we need earlier CKD detection, transparent communication about detection, tracking eGFR trajectory and rapid referral to nephrology including transplants.13 It was important to minimize bias and inaccuracy.
The group looked for an approach that would not disproportionately affect any one group of individuals, and found that of the five different approaches, CKD-epi using creatinine without the race coefficient was recommended because it included diversity during development as 31% of the participants within the CKD-EPI studies were identified as Black.13 It is also immediately available in all labs in the United States and does not disproportionately affect any group of individuals. While cystatin is not currently used, its routine use is encouraged as a combination of both creatinine and cystatin are more accurate and would support better clinical decisions.
In Delaware, significant headway has occurred as regards to eliminating the correction factor in 2022 as compared to 2021. In a phone survey to all the Delaware hospital systems conducted April 2022, many were moving to eliminate or had already eliminated the correction factor. Conversations were held with pathologists, laboratory supervisors, pharmacy, information technology and chemistry personnel; all were familiar with the eGFR race correction. While some systems have dropped the correction or are moving ahead in the process, two systems have no plans at present to eliminate it (see Table 1). The significance of this is unclear, especially considering the recommendation by the National Kidney Foundation-American Society of Nephrology.
Table 1. Status of eGFR Race Correction Factor Elimination.
| Hospital System | Status |
|---|---|
| Wilmington VA | In process of moving to eliminate the correction factor and should be completed soon. Working on computer testing. |
| ChristianaCare | Removed 4/12/22 |
| Nemours Children’s Health | No current plan to eliminate the correction factor |
| St. Francis Hospital/Trinity Health | No current plan to eliminate the correction factor |
| Tidal Health/Nanticoke | Working on eliminating the correction factor. No date in place, target: June/July 2022 |
| Beebe Healthcare | Removed 3/2022 |
| Bayhealth | Eliminating the correction factor late summer or fall |
Conclusion
Removal of race from eGFR is an opportunity for physicians to help reduce inequalities in treatment and outcomes. Instead of assuming biologic causes of health inequities, we can focus on social determinants of health that lead to disparities. Dr. Clive Callender, founder of the Howard University Hospital Transplant Center, once said “Institutionalized racism is the elephant in the room that has not been addressed. We have put this social construct, as enormous as it is, to become an obstacle to everything that we do, and we pay the price.” As current and future providers for many of these patients, we can help reduce these health inequities by advocating for our patients, looking at GFR when making clinical decisions, and supporting the elimination of race based GFR.
References
- 1.Witzig, R. (1996, October 15). The medicalization of race: Scientific legitimization of a flawed social construct. Annals of Internal Medicine, 125(8), 675–679. 10.7326/0003-4819-125-8-199610150-00008 [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, S., Nutt, C. T., Eneanya, N. D., Reese, P. P., Sivashanker, K., Morse, M., et al. Mendu, M. L. (2021, February). Examining the potential impact of race multiplier utilization in estimated glomerular filtration rate calculation on African-American care outcomes. Journal of General Internal Medicine, 36(2), 464–471. 10.1007/s11606-020-06280-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyriakopoulos, C., & Gupta, V. (2022, Jan). Renal failure drug dose adjustments. StatPearls. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK560512/ [PubMed]
- 4.Mula-Abed, W. A., Al Rasadi, K., & Al-Riyami, D. (2012, March). Estimated glomerular filtration rate (eGFR): A serum creatinine-based test for the detection of chronic kidney disease and its impact on clinical practice. Oman Medical Journal, 27(2), 108–113. 10.5001/omj.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey, A. S., Bosch, J. P., Lewis, J. B., Greene, T., Rogers, N., & Roth, D., & the Modification of Diet in Renal Disease Study Group . (1999, March 16). A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Annals of Internal Medicine, 130(6), 461–470. 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 6.Stenvinkel, P. (2010, November). Chronic kidney disease: A public health priority and harbinger of premature cardiovascular disease. Journal of Internal Medicine, 268(5), 456–467. 10.1111/j.1365-2796.2010.02269.x [DOI] [PubMed] [Google Scholar]
- 7.Kidneyfund.org. (2016). Kidney disease and African-Americans. https://www.kidneyfund.org/kidney-today/kidney-disease-african-americans.html
- 8.Turner, A. (2018). The business case for racial equity: A strategy for growth. W.K. Kellogg Foundation. https://altarum.org/sites/default/files/uploaded-publication-files/WKKellogg_Business-Case-Racial-Equity_National-Report_2018.pdf
- 9.Sharfstein, D. J. (2007). Crossing the color line: Racial migration and the one-drop rule, 1600-1860, 91. Minnesota Law Review, 592. Retrieved from http://scholarship.law.vanderbilt.edu/faculty-publications/386 [Google Scholar]
- 10.Yearby, R. (2021, February). Race based medicine, colorblind disease: How racism in medicine harms us all. Am J Bioeth, 21(2), 19–27. Epub2020Dec5. [DOI] [PubMed]
- 11.Jones, N., Marks, R., Ramirez, R., & Rios-Vargas, M. (2022). 2020 Census Illuminates Racial and Ethnic Composition of the Country. Census.gov. https://www.census.gov/library/stories/2021/08/improved-race-ethnicity-measures-reveal-united-states-population-much-more-multiracial.html
- 12.Bragg-Gresham, J., Zhang, X., Le, D., Heung, M., Shahinian, V., Morgenstern, H., & Saran, R. (2021, January 4). Prevalence of chronic kidney disease among Black individuals in the US after removal of the Black race coefficient from a glomerular filtration rate estimating equation. JAMA Network Open, 4(1), e2035636. 10.1001/jamanetworkopen.2020.35636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado, C., Baweja, M., Crews, D. C., Eneanya, N. D., Gadegbeku, C. A., Inker, L. A., et al. Powe, N. R. (2022, February). A unifying approach for GFR estimation: Recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis, 79(2), 268–288.e1. 10.1053/j.ajkd.2021.08.003 [DOI] [PubMed] [Google Scholar]