Abstract
Background and Objectives
Nursing home (NH) staff mealtime care approaches are associated with behaviors of residents with dementia, but their impact on food intake remains unexplored. This study examined the role of staff person-centered and task-centered approaches and resident positive, neutral, and challenging behaviors on food intake.
Research Design and Methods
Videotaped mealtime observations (N = 160) involving 36 staff and 27 residents (53 unique staff–resident dyads) in 9 NHs were coded using the refined Cue Utilization and Engagement in Dementia mealtime video-coding scheme. The dependent variable was resident food intake. The independent variables were staff person-centered approaches that support resident abilities, staff–resident (dyadic) interactions, and dining environments, staff task-centered approaches, and resident positive, neutral, and challenging behaviors. Resident challenging behaviors included mealtime functional impairments and resistive behaviors. Linear mixed modeling was used. Moderating effects of staff approaches, food type, and length of dyadic mealtime interactions (ie, video duration) were examined.
Results
The relationship between food intake and resident mealtime functional impairments was moderated by food type (p < .001). The relationship between food intake and resident resistive behaviors was moderated by food type (p = .002) and staff person-centered verbal approaches (p = .001). The relationships between food intake and staff person-centered nonverbal approaches (p = .003) and resident positive/neutral nonverbal behaviors (p = .004) were moderated by the length of dyadic mealtime interactions.
Discussion and Implications
Food intake was associated with staff person-centered approaches and resident positive/neutral and challenging behaviors. Findings emphasize the importance of facilitating positive dyadic interactions using individualized, context-based, multifaceted, person-centered care. Future research on temporal and causal relationships is warranted in larger diverse samples.
Keywords: Behavioral coding, Dyadic interaction, Person-oriented, Task-oriented, Video observations
Translational Significance: Staff person-centered approaches and resident positive/neutral and challenging behaviors have important impact on food intake. Such impact depends on mealtime contextual factors including food type being consumed and dyadic interaction length. Staff education on optimal dementia mealtime care is fundamental to (i) reframing their understanding of resident challenging behaviors from “negative behaviors to avoid/minimize” to “behaviors communicating needs and preferences that require attention and responses,” and (ii) increasing their skills and awareness to appropriately respond to resident challenging behaviors. The use of individualized, resident-centered, context-based strategies to respond to negative interactions and foster positive interactions is critical for quality mealtime care.
Background and Objectives
Eating is one of the most fundamental activities of daily living (ADLs) to maintain nutrition, function, and social engagement. People living with dementia in long-term care settings (residents) usually exhibit behavioral and psychological symptoms of dementia (BPSD), which interfere with mealtime and affect food intake (1,2). Inadequate food intake leads to health-related consequences including weight loss, increased risk of malnutrition and infection, and increased morbidity and mortality. All individual-level consequences may be associated with institutional outcomes such as increased psychotropic medication use, higher staffing needs, more staff stress, and turnover, all of which increase care costs (3–5). It is critical that optimal care is provided to respond to residents’ behaviors and ensure optimal mealtime experiences while minimizing the risks of rapid functional declines, inadequate food intake, and malnutrition.
Association of Person-Centered and Task-Centered Care and Resident Mealtime Behaviors With Food Intake
The Alzheimer’s Association highly recommends the use of person-centered care to support ADLs and BPSD in quality dementia care practice (6), indicating the significance of person-centered care to manage resident behaviors during mealtime. Person-centered care focuses on individualized care that engages and motivates residents in mealtime activities in an effort to address residents’ abilities, preferences, and needs (7,8). In contrast, task-centered care focuses on completing mealtime activities without adequate consideration of residents’ abilities, preferences, and needs (7,8). Mealtime is an important social event of everyday life that includes multiple stimuli such as meal-related items (eg, food, silverware, utensils), conversations, dining environment, staff, and other residents, all of which may trigger resident positive, neutral, and/or challenging behaviors (9,10). Residents may be positively engaged in the mealtime activities and/or social conversations, not responding to the stimuli such as provision of food or care, and/or exhibiting functional difficulties or resistiveness to care or food provided by staff (5).
Staff person-centered and task-centered care and resident positive, neutral, and challenging behaviors during mealtime were frequently observed and were significantly associated (9–11). Improved food intake is associated with person-centered mealtime care strategies, such as supporting resident independence in eating, offering liquid food when residents struggle with solid food, providing visual and physical assistance, and maintaining dyadic interactions and a quality dining environment (12–14). Resident agitation and risk of aspiration were more likely to occur following task-centered care than person-centered care during mealtime (15,16). However, the role of person-centered and task-centered care and resident behavioral responses on food intake remains understudied.
Eating is one of the most important ADLs that warrants person-centered, evidence-based care practice (6). It is important to understand how resident positive, neutral, and challenging behaviors affect food intake, as well as how caregiver person-centered and task-centered care influences the relationship between resident behaviors and food intake. This information will facilitate the creation and use of person-centered mealtime care interventions to respond to resident challenging behaviors, facilitate the transition of behaviors from challenging to positive and/or neutral, and further improve food intake.
Theoretical Framework
This study is guided by the integration of multiple theoretical models (Supplementary Figure 1), including the social ecological model (SEM) (17–19) and Kales et al.’s (20) conceptual model which combines important concepts from the consequences of need-driven, dementia-compromised behavior model (21,22) and the progressively lowered stress threshold model (23). The SEM describes the multilevel factors at the resident, caregiver, environmental, and institutional levels that are associated with resident behaviors and food intake during mealtime (1,5,14,24). By linking with the SEM, Kales et al.’s (20) conceptual model describes how interactions among multilevel factors lead to mealtime behaviors and subsequent consequences.
Neurodegenerations associated with dementia induce increased vulnerability to stressors and increased difficulties communicating needs during mealtime, which contributes to fluctuating behavioral states that are susceptible to challenging behaviors, and subsequent immediate, short-term, and long-term consequences such as inadequate food intake, declines in function and nutrition, and increased medication use. Stress can be increased or reduced through modifications of multilevel factors, which are potential modifiable targets of prevention and management efforts in dementia mealtime care practice.
Objectives
This study examined the role of staff person-centered and task-centered approaches and positive, neutral, and challenging mealtime behaviors in nursing home (NH) residents with dementia on food intake. It was hypothesized that staff person-centered approaches and resident positive/neutral behaviors were associated with more frequent food intake, and that staff task-centered approaches and resident challenging behaviors were associated with less frequent food intake.
Research Design and Methods
Study Design
This study was a secondary analysis of archived videotaped mealtime observations collected for a randomized clinical trial during 2011–2014. The parent study evaluated the efficacy of a dementia communication intervention to improve staff communication and decrease resident resistiveness to care (25,26). Ethical approvals from Institutional Review Boards of universities where the studies were conducted were obtained.
Sample and Setting
In the parent study, staff were eligible if they were: (i) at least 18-year-old, (ii) English speaking, (iii) permanent employees, and (iv) provided direct care for a participating resident ≥2 times/week over the previous month (25). Residents were eligible if they had: (i) dementia diagnosis based on medical records, (ii) long-stay status, (iii) staff-reported resistiveness to care, (iv) capacity to hear staff communication, and (v) a surrogate decision maker providing informed consent. A total of 127 staff and 83 residents from 13 NHs in Kansas, United States were enrolled in the parent study.
In this study, videotaped observations were selected from the parent study’s archived observations. Videos were included if they: (i) captured mealtime activities, (ii) lasted ≥1 minute, (iii) captured one-to-one interactions between 1 staff and 1 resident, and (iv) captured verbal and nonverbal behaviors with adequate video/audio quality. Videos were excluded if the resident was taking medication rather than eating a meal, being transferred to or from the dining location, or present in the dining location but not eating a meal. A total of 1 748 videos were screened, from which 1 588 videos were excluded due to not capturing mealtime activities (n = 1 486), lasting <1 minute (n = 63), involving multiple staff and/or residents (n = 34), and poor quality (n = 5), leaving 160 eligible videos included in this study. Among the 160 videos, 110 were collected prior to the dementia communication intervention (preintervention) and 50 post the intervention (postintervention).
Video Coding
The refined Cue Utilization and Engagement in Dementia (CUED) mealtime video-coding scheme was used to assess staff person-centered and task-centered approaches, resident positive, neutral, and challenging behaviors, and resident food intake process. Staff person-centered approaches were coded as 8 verbal behaviors (eg, giving choices) and 26 nonverbal behaviors that support resident abilities, dyadic interactions, and dining environments (eg, adjusting proximity). Staff task-centered approaches were coded as 4 verbal behaviors (eg, verbal refusal/disagreement) and 8 nonverbal behaviors (eg, outpacing).
Resident positive/neutral behaviors were coded as 8 verbal behaviors (eg, asking for help/cooperation) and 5 nonverbal behaviors (eg, wiping away oral spillage/drool). Resident challenging behaviors were coded as 4 verbal behaviors (eg, interrupting/changing topic) and 22 nonverbal behaviors demonstrating mealtime functional impairments (eg, difficulty using utensil properly) and resistive behaviors (eg, pushing away help/food).
An intake episode was defined as the process of transporting 1 bite of solid food or 1 drink of liquid food from the food container (eg, plate, cup, bowl) into the mouth. Four characteristics of each intake episode were coded: (i) the person who initiated and completed each episode (ie, resident, staff), (ii) the type of food being consumed (solid, liquid), (iii) the starting and ending time points, and (iv) the outcome (intake, no intake).
All videotaped observations were coded second-by-second by 1 of 4 trained coders during 2018–2020 using Noldus Observer 14.0 (Noldus Information Technology Inc., Leesburg, VA). All behavioral codes and their operational definitions, as well as the process of training coders and video coding were described in detail elsewhere (10,12,13,26). The refined CUED shows adequate evidence for ease of use, feasibility, interrater reliability (Cohen’s kappa range = 0.93–0.99, 95% confidence interval = 0.92–0.99, ±1s tolerance), construct validity, and predictive validity using videotaped mealtime interactions (10,12,13,26,27). Coded data were exported from Noldus Observer to Excel worksheets, and then to SAS 9.4 (28).
Variables
Participant characteristics
Participant characteristics were collected in the parent study. Resident characteristics included age, gender, race, ethnicity, dementia stage, functional disability, and physical comorbidities. Dementia stage was determined by extracting data on Functional Assessment Staging in Alzheimer’s disease (ranging from 1, normal cognition/functioning, to 8, very severe dementia) from Minimum Data Set (MDS) 3.0 (29). Functional disability (ADL self-performance and support provided) was extracted from MDS 3.0 Section G (total score ranges from 0 to 160, higher score = more dependence in self-performance and more support needed). Physical comorbidities were evaluated by reviewing MDS 3.0 and clinical records using the Modified Cumulative Illness Rating Scale (total score ranges from 0 to 70, higher score = more comorbidities) (30). Staff characteristics included age, gender, race, ethnicity, education, job title, number of years worked as a nursing caregiver, and number of years worked in the current facility.
Length of dyadic mealtime interactions
The length of dyadic mealtime interactions was the period of time that the resident spent in mealtime activities and the staff spent providing care in each videotaped observation (ie, video duration, in minutes). Each video used in the study captured 1 individual resident’s mealtime activities assisted by 1 staff and all videos had varied lengths of dyadic mealtime interactions.
Resident eating function
Resident eating function was conceptualized as the level of resident functional independence to initiate and complete food intake episodes, and was operationalized as the proportion of intake episodes initiated and completed by a resident (ie, the total number of intake episodes initiated and completed by a resident divided by the total number of intake episodes in all videos that involved the same resident). Based on the distribution of eating function, residents were categorized as dependent (0% to 25%), partially (in)dependent (>25% to <75%), and independent (75% to 100%).
Resident food intake
Resident food intake was operationalized as the number of intake episodes that resulted in successful intake of solid or liquid food per minute. An indicator variable, food type, was added to the data to identify the number of intake episodes per minute for solid food and liquid food. The per-minute adjustment was used to account for varied length of dyadic mealtime interactions (ie, video duration). Thus, food intake was calculated with 2 values for each video as follows:
The number of intakes of solid food/minute equals the total number of episodes that result in intake of solid food divided by video duration.
The number of intakes of liquid food/minute equals the total number of episodes that result in intake of liquid food divided by video duration.
Resident positive and neutral behaviors
Resident positive verbal behaviors were defined as verbal behaviors of residents that indicate engagement or cooperation in eating (eg, asking for help/cooperation) and operationalized as the number of resident positive utterances observed in each video divided by video duration and categorized as the number of behaviors per minute: 0, between 0 and 1, and 1 or more.
Resident positive/neutral nonverbal behaviors were defined as nonverbal behaviors of residents that indicate engagement or autonomy in eating (eg, wiping away oral spillage/drool) and operationalized as the number of resident nonverbal positive or neutral behaviors observed in each video divided by video duration and categorized as the number of behaviors per minute: 0, between 0 and 1, and 1 or more. Resident nonverbal behaviors that were positive and neutral were grouped together because limited number of positive and neutral nonverbal behaviors was observed in the video sample.
Resident challenging behaviors
Resident mealtime functional impairments were defined as decline in, or loss of, functional abilities related to mealtime activities and the intake process, and operationalized as the number of resident nonverbal behaviors indicating chewing or swallowing difficulties, or other functional difficulties in transporting food from the container to the mouth (eg, difficulties using utensil/hand properly) observed in each video divided by video duration and categorized as the number of behaviors per minute: 0, between 0 and 1, and 1 or more.
Resident resistive behaviors were defined as verbal and nonverbal challenging behaviors that indicate refusal of, or resistiveness to, care and/or food provided by staff and operationalized as the number of resident verbal (eg, interrupting/changing topic) or nonverbal (eg, pushing away help/food) negative behaviors that indicate refusal of, or resistiveness to, care or food observed in each video divided by video duration and categorized as the number of behaviors per minute: 0, between 0 and 1, and 1 or more.
Staff person-centered approaches
Staff person-centered verbal approaches were defined as verbal assistance provided by staff to engage and motivate residents during mealtime by accommodating resident cognitive and functional abilities, dyadic interaction approaches, and physical and social dining environments, and operationalized as the number of staff person-centered utterances (eg, giving choices) divided by video duration.
Staff person-centered nonverbal approaches were defined as nonverbal assistance provided by staff to engage and motivate residents during mealtime by accommodating resident cognitive and functional abilities, dyadic interaction approaches, and physical and social dining environments, and operationalized as the number of staff nonverbal person-centered behaviors (eg, adjusting proximity) divided by video duration.
Staff task-centered approaches
Staff task-centered approaches were defined as verbal and nonverbal assistance provided by staff that focuses on completing mealtime tasks rather than accommodating resident abilities, needs, and preferences, and operationalized as the number of staff negative verbal (eg, verbal refusal/disagreement) or nonverbal (eg, outpacing) approaches in each video divided by video duration and categorized as the number of behaviors per minute: 0, between 0 and 1, and 1 or more.
Data Analysis
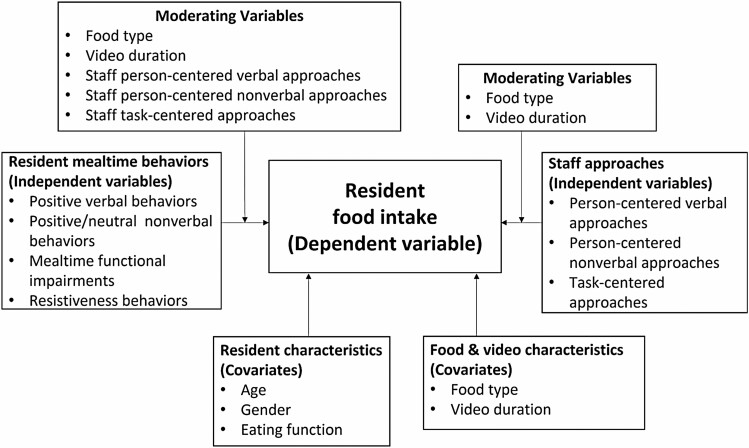
All statistical analyses were performed in SAS 9.4 (28). The level of significance was set at alpha = 0.05. Characteristics of participants and videos were summarized using descriptive statistics. Linear mixed modeling (LMM) with the residual maximum likelihood estimation method was used to examine the role of staff person-centered and task-centered approaches and resident positive, neutral, and challenging behaviors (7 independent variables) on food intake (1 dependent variable) (31). Figure 1 displays the variables and their relationships tested in the model. A compound symmetry covariance structure was used to account for within-video correlation due to repeated measures (ie, food intake was measured twice for each video as the number of intakes of solid food/minute and the number of intakes of liquid food/minute, indicated by the variable “food type”). Dyads were fit as a random effect to account for clustering within dyads because the same dyads were seen in multiple videos. The covariance parameter estimate for the random effect of dyads was not statistically significant (p = .421). Residuals were examined graphically to identify violations to normality and homogeneity of variance assumptions, as well as outliers and influential observations (31).
Figure 1.
Variables and relationships tested in the models.
For LMM analysis, the number of intakes of solid and liquid food/minute (dependent variable) was transformed based on the examination of residuals as follows: (i) a constant 1 was added to each value to eliminate zero intake, and (ii) the natural log function was applied to the values. Two-way interaction effects of each of the 7 independent variables with food type (ie, solid, liquid) and the length of dyadic mealtime interactions (ie, video duration) were examined, because food type and video duration were associated with food intake in prior work (13) and may moderate the relationships of food intake with resident behaviors and staff approaches. Two-way interaction effects of the 4 independent variables representing resident behaviors with the 3 independent variables representing staff approaches were also examined, because staff approaches were associated with resident behaviors and food intake in prior work (9–12), and may moderate the relationships between resident behaviors and food intake. Due to the relatively small sample size, a sequential approach was used to test interaction effects, and only significant interaction effects (p < .05) were included in the model. The 7 independent variables were included in the model regardless of their significance.
Covariates included in the model as fixed effects were resident age, gender, and eating function because these characteristics were associated with food intake in prior work (5,14,32), and length of dyadic mealtime interactions due to varying video durations. For ease of interpretation, continuous covariates (resident age, length of dyadic mealtime interactions) were centered at the sample means. The length of dyadic mealtime interactions was natural log-transformed prior to centering. The effect of the dementia communication intervention (preintervention vs postintervention) was not significant (p = .583) and, thus, not included in the model.
Results
Participant Characteristics
The 160 videos involved 27 residents and 36 staff (53 unique staff–resident dyads) in 9 NHs. Resident participants had a mean age of 85.6 years (Supplementary Table 1). All residents were White. The majority were female (63.0%) and non-Hispanic (92.6%). Residents had moderately severe (70.0%) or severe (30.0%) dementia, and moderate levels of functional disability (range = 12–39) and physical comorbidities (range = 19–36). Regarding eating function, 37.0% of the residents were independent, 40.8% were partially (in)dependent, and 22.2% were dependent on mealtime assistance. Staff participants had a mean age of 35.9 years (Supplementary Table 2), worked as a caregiver for a mean length of 9.5 years, and worked at the current NH for a mean length of 4.0 years. Most staff were female (80.6%), non-Hispanic (75.0%), and White (75.0%), had completed or were attending college (72.2%), and were certified nursing assistants (85.7%).
Video Characteristics
The length of dyadic mealtime interactions in the video sample was 4.5 minutes (Supplementary Table 3). The mean number of intakes of solid and liquid food/minute was 1.3 (range = 0–7.2) and 0.9 (range = 0–5.4), respectively. The mean number of staff person-centered nonverbal and verbal approaches/minute was 3.6 (range = 0–13.6) and 4.4 (range = 0–13.4), respectively. Staff task-centered approaches were observed in 76.2% of the videos. Resident positive verbal behaviors, positive/neutral nonverbal behaviors, mealtime functional impairments, and resistive behaviors were observed in 68.1%, 55.6%, 83.1%, and 71.9% of the videos, respectively.
Association of Food Intake With Resident Mealtime Behaviors and Staff Person-Centered and Task-Centered Approaches
Five videos that captured 1 resident with missing data on age were excluded, resulting in 155 videos (26 residents and 35 staff) in the final model (Table 1). Staff task-centered approaches (verbal and nonverbal combined, p = .534) and resident positive verbal behaviors (p = .490) were not associated with food intake. Staff person-centered verbal approaches moderated the relationship between food intake and resident resistive behaviors. The relationships between food intake and the other independent variables (ie, staff person-centered nonverbal approaches, resident positive/neutral nonverbal behaviors, resident mealtime functional impairments, resident resistive behaviors) were moderated by food type, length of dyadic mealtime interactions, and/or staff person-centered verbal approaches, as indicated by 5 significant interaction effects (Supplementary Figures 2–5).
Table 1.
Linear Mixed Model for Intake per Minute (natural log-transformed)
| Variable | b [95% CI] | t | p * | F | p † |
|---|---|---|---|---|---|
| Intercept | 0.60 [0.37, 0.83] | 5.17 | <.001 | ||
| Resident positive verbal behaviors/min | 0.72 | .490 | |||
| 1 or more vs 0 | −0.06 [−0.18, 0.05] | −1.11 | .271 | ||
| Between 0 and 1 vs 0 | −0.05 [−0.14, 0.05] | −0.95 | .343 | ||
| Resident positive/neutral nonverbal behaviors/min | 1.74 | .179 | |||
| 1 or more vs 0 | 0.08 [−0.04, 0.21] | 1.29 | .201 | ||
| Between 0 and 1 vs 0 | 0.08 [−0.01, 0.17] | 1.78 | .077 | ||
| Resident positive/neutral nonverbal behaviors/min × Length of dyadic mealtime interactions [ln(min)] | 5.67 | .004 | |||
| 1 or more vs 0 | 0.13 [−0.05, 0.31] | 1.40 | .164 | ||
| Between 0 and 1 vs 0 | −0.15 [−0.27, −0.02] | −2.37 | .019 | ||
| Resident mealtime functional impairments (nonverbal)/min | 2.42 | .093 | |||
| 1 or more vs 0 | 0.26 [0.08, 0.43] | 2.80 | .006 | ||
| Between 0 and 1 vs 0 | 0.10 [−0.08, 0.29] | 1.11 | .269 | ||
| Resident mealtime functional impairments (nonverbal)/min × food type | 8.44 | <.001 | |||
| Liquid vs solid food (1 or more vs 0) | −0.58 [−0.86, −0.30] | −4.11 | <.001 | ||
| Liquid vs solid food (between 0 and 1 vs 0) | −0.43 [−0.72, −0.15] | −2.98 | .003 | ||
| Resident resistive behaviors (verbal and nonverbal)/min | 0.32 | .727 | |||
| 1 or more vs 0 | −0.06 [−0.30, 0.17] | −0.54 | .592 | ||
| Between 0 and 1 vs 0 | −0.16 [−0.36, 0.03] | −1.67 | .097 | ||
| Resident resistive behaviors (verbal and nonverbal)/min × food type | 6.50 | .002 | |||
| Liquid vs solid food (1 or more vs 0) | 0.28 [0.01, 0.55] | 2.01 | .046 | ||
| Liquid vs solid food (between 0 and 1 vs 0) | 0.43 [0.19, 0.67] | 3.60 | <.001 | ||
| Resident resistive behaviors (verbal and nonverbal)/min × staff person-centered verbal approaches/min | 7.13 | .001 | |||
| 1 or more vs 0 | −0.07 [−0.11, −0.03] | −3.78 | <.001 | ||
| Between 0 and 1 vs 0 | −0.04 [−0.08, −0.01] | −2.43 | .016 | ||
| Staff person-centered nonverbal approaches/min | 0.04 [0.02, 0.07] | 3.51 | .001 | 12.34 | .001 |
| Staff person-centered nonverbal approaches/min × Length of dyadic mealtime interactions [ln(min)] | 0.04 [0.01, 0.06] | 3.09 | .003 | 9.54 | .003 |
| Staff person-centered verbal approaches/min | 0.03 [0.00, 0.06] | 2.06 | .042 | 0.37 | .544 |
| Staff task-centered approaches (verbal and nonverbal)/min | 0.63 | .534 | |||
| 1 or more vs 0 | −0.07 [−0.21, 0.06] | −1.05 | .294 | ||
| Between 0 and 1 vs 0 | −0.01 [−0.09, 0.08] | −0.12 | .901 | ||
| Food type (liquid vs solid food) | −0.06 [−0.33, 0.20] | −0.48 | .634 | 9.29 | .003 |
| Length of dyadic mealtime interactions [ln(min)] | −0.03 [−0.16, 0.11] | −0.40 | .691 | 0.31 | .581 |
| Resident age (yr) | −0.01 [−0.02, 0.00] | −3.36 | .001 | 11.29 | .001 |
| Resident gender (female vs male) | −0.20 [−0.31, −0.10] | −4.04 | .001 | 16.30 | .001 |
| Resident eating function | 6.61 | .008 | |||
| Partially (in)dependent vs dependent | 0.21 [0.07, 0.35] | 3.30 | .006 | ||
| Independent vs dependent | 0.05 [−0.08, 0.18] | 0.76 | .450 |
Notes: N = 155 videos. Resident positive verbal behaviors/min = number of resident positive utterances, per minute. Resident positive/neutral nonverbal behaviors/min = number of resident nonverbal positive or neutral behaviors, per minute. Resident mealtime functional impairments (nonverbal)/min = number of resident nonverbal behaviors indicating chewing or swallowing difficulties, or other functional difficulties in transporting food from the container to the mouth, per minute. Resident resistive behaviors/min = number of resident verbal or nonverbal negative behaviors that indicate resistiveness to care or food per minute. Staff person-centered verbal approaches/min = number of person-centered utterances made by staff towards residents, per minute. Staff person-centered nonverbal approaches/min = number of staff nonverbal person-centered behaviors that support resident abilities, dyadic interactions, and physical and social dining environments, per minute. Staff task-centered approaches (verbal and nonverbal)/min = number of staff negative verbal or nonverbal behaviors, per minute. Resident eating function = proportion of food intake episodes initiated by resident: independent = 75%–100% of food intake episodes initiated by resident; partially (in)dependent = between 25% and 75% of food intake episodes initiated by resident; dependent = 0%–25% of food intake episodes initiated by resident. b = unstandardized regression coefficient estimate; 95% CI = 95% confidence interval.
*p Values for a t test of significance of an effect or a level of an effect.
† p Values for an F-test of significance of each effect overall.
The relationship between food intake and resident mealtime functional impairments was moderated by food type (p < .001; Supplementary Figure 2). When residents consumed liquid food, residents who exhibited mealtime functional impairments had fewer intakes/minute, compared to residents who did not exhibit mealtime functional impairments. When residents consumed solid food, those residents who had more intakes/minute exhibited more mealtime functional impairments/minute.
The relationship between food intake and resident resistive behaviors was moderated by food type (p = .002) and staff person-centered verbal approaches (p = .001; Supplementary Figure 3). For residents who did not exhibit resistive behaviors, the number of intakes of solid and liquid food/minute increased as staff person-centered verbal approaches increased. However, for residents who exhibited resistive behaviors, the number of intakes of solid and liquid food/minute decreased as staff person-centered verbal approaches increased. Additionally, intake of liquid food/minute was higher for residents who exhibited between 0 and 1 resistive behaviors/minute, compared to residents who exhibited 1 or more resistive behaviors/minute, while intake of solid food/minute was more similar for these 2 groups of residents.
The relationship between food intake and resident positive/neutral nonverbal behaviors was moderated by length of dyadic mealtime interactions (p = .004; Supplementary Figure 4). Food intake/minute was about the same for residents who exhibited between 0 and 1 positive/neutral nonverbal behaviors/minute in the video. As the length of videotaped dyadic mealtime interactions increased, food intake/minute increased somewhat for residents who exhibited no positive/neutral nonverbal behaviors/minute and increased comparatively more for residents who exhibited 1 or more positive/neutral nonverbal behaviors/minute.
The relationship between food intake and staff person-centered nonverbal approaches was moderated by length of dyadic mealtime interactions (p = .003; Supplementary Figure 5). As the number of staff person-centered nonverbal approaches/minute increased, food intake/minute also increased. This relationship was more pronounced for longer (vs shorter) lengths of dyadic mealtime interactions.
Resident age, gender, and eating function, as covariates controlled for in the model, were associated with the number of intakes of solid and liquid food/minute. Older residents had fewer intakes/minute than younger residents (p = .001). Female residents had fewer intakes/minute than male residents (p = .001). Residents who were dependent on mealtime assistance had fewer intakes/minute than partially (in)dependent residents (p = .006). There was no difference in food intake between residents independent versus dependent on mealtime assistance (p = .450).
Discussion and Implications
This is the first study that examined the role of resident mealtime behaviors and staff person-centered and task-centered approaches on food intake using the refined CUED and videotaped observations. This study identified the association of food intake with staff person-centered approaches and resident mealtime positive/neutral and challenging behaviors. In addition, food type, length of dyadic mealtime interactions, and/or staff person-centered verbal approaches moderated these relationships. This study did not identify any association of food intake with staff task-centered approaches and resident positive verbal behaviors.
Resident Mealtime Functional Impairments (Nonverbal) and Food Type
The association between food intake and resident mealtime functional impairments depended on food type. When residents had more intakes of solid food (vs liquid food), they exhibited more functional impairments, including difficulties with chewing and swallowing, and difficulties using utensils and transporting food into the mouth. While consumption of both solid and liquid food involves swallowing efforts, consumption of solid (vs liquid) food is more likely to involve use of utensils to transport food and continuing chewing activities which require more functional and physical efforts, and thereby may be associated with more observable, behavioral indicators of functional impairments. Findings support previously reported mealtime care strategies including offering drinks regularly to ensure hydration between bites of solid food when residents can tolerate liquid-textured food (13). In addition, modified texture diets are often provided to residents who demonstrate difficulties with chewing solid food (eg, texture-modified food including soft, minced, pureed food) and/or swallowing liquid food (eg, mildly to extremely thickened fluids) (33). Further, despite exhibition of functional impairments, the attempts to eat solid food increased the number of actual intakes of solid food, suggesting that resident independence in eating with or without assistance should be encouraged. Functional impairments may be an indicator that residents struggle with the intake process and need staff assistance to continue eating, rather than conveying “stop,” “refusal,” or “resistiveness.” Staff assistance may play a role in the relationship between food intake and resident functional impairments. While this study failed to identify any significant interactions between staff approaches and resident functional impairments, future research is warranted to understand the role of staff approaches on food intake when residents exhibit functional impairments.
Resident Resistive Behaviors and Staff Person-Centered Verbal Approaches by Food Type
The association between food intake and resident resistive behaviors depended on food type and staff person-centered verbal approaches. For residents who did not exhibit resistive behaviors, intake of both liquid and solid food was similar and increased as staff person-centered verbal approaches increased. For residents who exhibited resistive behaviors, intake of liquid food/minute was higher for residents who exhibited between 0 and 1 resistive behaviors/minute, compared to residents who exhibited 1 or more resistive behaviors/minute, while intake of solid food/minute was more similar for the 2 groups of residents. Further, staff person-centered verbal approaches were associated with less intake of both liquid and solid food for residents who exhibited resistive behaviors. Possibly, residents who were more compliant were more responsive to staff person-centered verbal cues which facilitated food intake. However, staff person-centered verbal cues were observed along with lower intake for those residents who exhibited resistive behaviors, possibly indicating that positive verbal cues from staff may not be helpful or adequate to overcome resident resistive behaviors during mealtime.
Resident Positive/Neutral Nonverbal Behaviors and Staff Person-Centered Nonverbal Approaches With the Length of Dyadic Mealtime Interactions
The associations of food intake with staff person-centered nonverbal approaches and resident positive/neutral nonverbal behaviors were moderated by length of dyadic mealtime interactions. Residents who received more staff person-centered nonverbal approaches/minute had more food intake when lengths of dyadic mealtime interactions were longer (vs shorter). Residents who exhibited 1 or more positive/neutral behaviors had more food intakes/minute compared to residents who exhibited fewer than 1 positive/neutral behaviors/minute in longer (vs shorter) dyadic mealtime interactions. While the video sample captured segments of meals rather than entire meals, findings support the importance of fostering positive, person-centered, continuous dyadic interactions in engaging residents in eating and improving food intake, consistent with prior work (13).
Implications for Practice
This study supported the role of staff person-centered approaches and resident positive/neutral and challenging behaviors on food intake and these relationships can be complex and context-dependent. While the video sample captured segments of meals rather than entire meals, it reflects usual NH mealtime care practice, because the average length of the video sample (4.5 ± 3.8 minutes) was consistent with previously reported actual amount of staff time spent providing mealtime assistance (averaged 5 to 9 minutes per resident per meal) (34–37). The study findings have potential generalizability and impact on current NH mealtime care practice. Additionally, the study focus aligns well with multiple Alzheimer’s Association Dementia Care Practice Recommendations (6), including person-centered care (7), information, education, and support for individuals living with dementia and their caregivers (38), care of BPSD (39), support of ADLs (8), Staffing (40), and supportive and therapeutic environments (41).
First, findings reframe the understanding of resident mealtime challenging behaviors from “negative behaviors to avoid or minimize” to “behavioral responses that communicate needs and preferences that require staff attention and responses as part of the mealtime care routine.” Resident initiation of intake episodes with or without staff assistance should be strongly encouraged, even when residents exhibit challenging behaviors including functional impairments or resistive behaviors, which are commonly observed when residents attempt to consume solid or liquid food. The impact of resident challenging behaviors on food intake is not always negative (eg, decreasing food intake) and is dependent on food type. For example, mealtime functional impairments are not negative behaviors that inhibit the intake process, but an indicator that residents struggle with the intake process and need appropriate staff assistance to facilitate successful intake.
Second, findings emphasize the importance of increasing awareness of and providing appropriate responses to resident verbal and, more importantly, nonverbal behaviors during mealtime. All resident nonverbal behaviors including positive/neutral behaviors, mealtime functional impairments, and resistive behaviors were associated with food intake, indicating the importance of being aware of and continuously monitoring resident nonverbal behaviors. Residents with advanced dementia experience decline or loss of ability to communicate needs and preferences verbally and instead rely on nonverbal communication. Prior work reported two thirds of resident mealtime behaviors were nonverbal (9,10). Continuous, positive, nonverbal interactions among the dyad are an important strategy to facilitate resident positive/neutral nonverbal behaviors and food intake.
Third, findings inform the use of individualized, resident-centered, context-based, mealtime care strategies to foster positive and neutral interactions. It is highly recommended that person-centered mealtime care attend to “resident dignity, respect and choice; the dining process and environment; health and biological considerations; adaptations and functioning; and food, beverage and appetite” (8). The different role of staff person-centered verbal versus nonverbal approaches may be considered: while person-centered nonverbal approaches are associated with more food intake, person-centered verbal approaches are associated with improved food intake among residents without resistive behaviors and are associated with decreased food intake among residents with resistive behaviors. Findings indicate person-centered care may not be “one size fit all.” The use of person-centered verbal versus nonverbal approaches needs to be personalized and context-dependent based on the understanding of residents’ needs and preferences through their verbal and nonverbal behavioral responses. For example, for residents who do not exhibit resistive behaviors, staff may offer more person-centered verbal approaches to improve food intake; while for residents with resistive behaviors, staff need to understand and address the causes of resistive behaviors, talk less (even in a positive or person-centered way), and/or provide more nonverbal approaches such as offering drinks regularly or modifying dining environment.
Implications for Research
Findings have important implications for the development and evaluation of dementia mealtime care interventions. First, resident positive/neutral and challenging behaviors have direct impact on food intake, and staff person-centered approaches may moderate these relationships. Therefore, staff person-centered mealtime care training that focuses on responding to resident positive/neutral and challenging behaviors seems logical and feasible to improve food intake. It is important that the development of innovative mealtime care interventions focus on not only encouraging staff to provide more person-centered approaches, but also appropriate use of person-centered approaches to respond to challenging behaviors and foster positive/neutral behavioral responses in residents based on mealtime contexts (eg, type of food being consumed, remaining time for meals). For example, while both staff person-centered verbal and nonverbal approaches are associated with improved food intake, person-centered verbal approaches seem more useful to residents without resistive behaviors and may inhibit food intake when provided to residents with resistive behaviors. Staff person-centered nonverbal approaches seem to be associated with more food intake whether residents show resistive behaviors or not.
Second, it is important to consider staff person-centered versus task-centered approaches as well as resident positive/neutral versus challenging behaviors separately in evaluating the process, fidelity, and effects of mealtime care interventions. Additionally, it is important and necessary to consider verbal and nonverbal behaviors separately. For example, this study showed different impacts of staff person-centered verbal and nonverbal approaches on intake in mealtime contexts. Also, resident positive/neutral and challenging behaviors associated with intake were mostly nonverbal (eg, positive/neutral behaviors and mealtime functional impairments were all nonverbal behaviors; resistive behaviors included both verbal and nonverbal behaviors), and resident positive verbal behaviors were not associated with intake. Findings indicate resident nonverbal behaviors, whether positive/neutral or challenging, may be important outcomes in evaluating effects of mealtime care interventions.
Third, findings guide our next step in developing and evaluating the Optimizing Mealtime Care (OPTIMAL) intervention, which features individualized, multifaceted, and individual-oriented mealtime care through adherence of the RECIPE principles: (i) Showing Respect, (ii) Creating Environment, (iii) Offering Choices, (iv) Supporting Independence, (v) Acknowledging Preferences, and (vi) Maintaining Engagement (5,10,13,42). The OPTIMAL intervention will be evidence-based and theoretically grounded, targeting the multilevel aspects of the theoretical framework used in this study: the impact of staff person-centered mealtime care on resident behavioral responses, function, and food intake through modifications of resident-level factors (cognitive and functional abilities), caregiver-level factors (dyadic interactions, social engagement), and environment/institutional-level factors (physical and social environments, care practice). This effort will address an important gap in mealtime care intervention research, because most existing behavioral interventions that aim to address BPSD are sensory practices, psychosocial practices, and structured care practices, and none focuses on education and support for caregivers on the management of BPSDs during mealtime using person-centered care for dementia populations (39).
Future Research Directions
This study pointed out 4 directions for future dementia mealtime care research. First, staff person-centered and task-centered approaches, resident mealtime behaviors, and food intake were operationalized in specific ways as continuous (number per minute) or categorical variables based on their distributions in this study. Future research is needed to verify the findings in diverse, larger samples. Second, while this study found all resident nonverbal behaviors, from positive/neutral to challenging, were associated with intake, the relationship between resident positive verbal behaviors and intake was not detected. Future research is needed to examine the role of resident positive verbal behaviors on food intake. Third, this study explored the moderating role of staff care approaches on the relationship of intake and resident behaviors. While the study showed some evidence of the moderating role of staff person-centered care approaches, staff task-centered care approaches were not associated with resident food intake and did not moderate the relationship between food intake and resident behaviors. While practice recording sessions were conducted prior to formal recordings in the parent study to minimize the Hawthorne effect by allowing participants to adjust to the novelty of being recorded, be familiar with the videographer’ faces and existence, and behave more naturally during formal recordings, participants were notified and aware of video recording and may not perform the same as they were unobserved. The videos captured limited variations of staff task-centered verbal and nonverbal approaches and further investigation on the role of staff task-centered care approaches in more diverse samples is needed. Lastly, this study focused on associations of food intake with staff approaches and resident behaviors as well as the moderating role of staff approaches on these associations using cross-sectional data. Future research may examine the temporal and/or causal relationships between staff person- and task-centered approaches and resident behaviors, and between staff–resident interactions and resident food intake.
Limitations
The video sample captured segments of meals rather than entire meals, certain characteristics of food intake process and dyadic interactions instead of other mealtime contextual factors (eg, texture-modified food, physical environment), and one-to-one rather than more complex (eg, 1:2, 2:1) interactions. Models controlled for clustering effects by dyad, rather than by resident, staff, and NH. Resident neutral behaviors were not separately analyzed due to limited codes and variation of data. Participants were NH direct care staff and residents with moderately severe to severe dementia who had exhibited resistiveness to care, limiting the generalizability of findings to similar long-term care staff and resident populations.
Conclusion
This study provided preliminary evidence to support the role of resident positive/neutral and challenging behaviors as well as staff person-centered approaches on resident food intake. Findings advance the understanding of resident positive/neutral and challenging behaviors as a way to communicate needs and preferences, emphasize the importance of transitioning challenging interactions to positive/neutral and fostering such positive/neutral interactions, and facilitate the use of individualized, context-based, resident-centered approaches. Future research is warranted to examine temporal or causal relationships in larger diverse samples.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Kristine Williams for providing access to the data collected in the parent study, technical support on Noldus Observer XT coding, as well as advice on manuscript revision. The authors acknowledge the research assistants for coding the videos in this study. We acknowledge the contribution of Dr. Melissa Batchelor in developing and refining coding scheme (CUED), which was used in this study for data coding, and providing support in the data coding process. The authors also acknowledge that the results of this study were previously presented at the 2021 National Hartford Center of Gerontological Nursing Excellence (NHCGNE) Virtual Leadership Conference (poster presentation) and the 2022 Mid-West Nursing Research Society (MNRS) 46th Annual Research Conference (competitive symposium).
Contributor Information
Wen Liu, The University of Iowa College of Nursing, Iowa City, Iowa, USA.
Yelena Perkhounkova, The University of Iowa College of Nursing, Iowa City, Iowa, USA.
Maria Hein, The University of Iowa College of Nursing, Iowa City, Iowa, USA.
Funding
This work was supported by National Institute of Aging (NIA) at National Institute of Health (NIH) [R03AG063170], and American Nurses Foundation Nursing Research Grant (PI: W. Liu). W. Liu was also supported by a Career Development Award from NIH/NIA [K23AG066856] for preparation of this manuscript. The parent study was supported by National Institute of Nursing Research (NINR) at National Institute of Health (NIH) [NR011455-04], Changing Talk to Reduce Resistiveness in Dementia Care (CHAT), PI: Kristine Williams. ClinicalTrials.gov Identifier: NCT01324219. The sponsors were not involved in study design, data collection and analysis, interpretation of findings, and manuscript preparation.
Conflict of Interest
None declared.
Author Contributions
W. Liu contributed to study design, video screening and coding, data analysis and synthesis, and writing and revision of the manuscript. Y. Perkhounkova contributed to data analysis, interpretation of findings, and manuscript revision. M. Hein contributed to data cleaning and management, data analysis, and manuscript revision. All authors meet the criteria for authorship and have approved the final draft submitted. All those entitled to authorship are listed as authors.
References
- 1. Liu W, Galik E, Boltz M, et al. . Factors associated with eating performance for long‐term care residents with moderate‐to‐severe cognitive impairment. J Adv Nur. 2016;72(2):348–360. doi: 10.1111/jan.12846 [DOI] [PubMed] [Google Scholar]
- 2. Chang CC. Prevalence and factors associated with feeding difficulty in institutionalized elderly with dementia in Taiwan. J Nutr Health Aging. 2012;16(3):258–261. doi: 10.1007/s12603-011-0158-6 [DOI] [PubMed] [Google Scholar]
- 3. Bell CL, Lee AS, Tamura BK. Malnutrition in the nursing home. Curr Opin Clin Nutr Metab Care. 2015;18(1):17–23. doi: 10.1097/MCO.0000000000000130 [DOI] [PubMed] [Google Scholar]
- 4. Hanson LC, Ersek M, Lin FC, et al. . Outcomes of feeding problems in advanced dementia in a nursing home population. J Am Geriatr Soc. 2013;61(10):1692–1697. doi: 10.1111/jgs.12448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu W, Tripp-Reimer T, Williams K, et al. . Facilitators and barriers to optimizing eating performance among cognitively impaired older adults: a qualitative study of nursing assistants’ perspectives. Dementia. 2020;19(6):2090–2113. doi: 10.1177/1471301218815053 [DOI] [PubMed] [Google Scholar]
- 6. Fazio S, Pace D, Maslow K, et al. . Alzheimer’s Association Dementia Care Practice Recommendations. Oxford University Press US; 2018. doi: 10.1093/geront/gnx182 [DOI] [PubMed] [Google Scholar]
- 7. Fazio S, Pace D, Flinner J, et al. . The fundamentals of person-centered care for individuals with dementia. Gerontologist. 2018;58(suppl_1):S10–S19. doi: 10.1093/geront/gnx122 [DOI] [PubMed] [Google Scholar]
- 8. Prizer LP, Zimmerman S. Progressive support for activities of daily living for persons living with dementia. Gerontologist. 2018;58(suppl_1):S74–S87. doi: 10.1093/geront/gnx103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu W, Perkhounkova Y, Williams K, et al. . Mealtime nonverbal behaviors in nursing home staff and residents with dementia: behavioral analyses of videotaped observations. Geriat Nurs. 2022;44:112–124. doi: 10.1016/j.gerinurse.2022.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu W, Williams K, Batchelor M, et al. . Mealtime verbal interactions among nursing home staff and residents with dementia: a secondary behavioural analysis of videotaped observations. J Adv Nurs. 2021;43(4):374–380. doi: 10.1093/geroni/igaa057.1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu W, Williams KN, Batchelor MK, Perkhounkova E, Hein M. Person-centered & task-centered mealtime care: impact on positive, neutral, & challenging behaviors in people with dementia. The Gerontological Society of America (GSA)’s 73rd Annual Scientific Meeting (virtual); 2021. Paper presentation. [Google Scholar]
- 12. Liu W, Perkhounkova E, Williams K, et al. . Food intake is associated with verbal interactions between nursing home staff and residents with dementia: a secondary analysis of videotaped observations. Int J Nurs Stud. 2020;109:103654. doi: 10.1016/j.ijnurstu.2020.103654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu W, Williams K, Batchelor-Murphy M, et al. . Eating performance in relation to intake of solid and liquid food in nursing home residents with dementia: a secondary behavioral analysis of mealtime videos. Int J Nurs stud. 2019;96:18–26. doi: 10.1016/j.ijnurstu.2018.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu W, Jao YL, Williams KN. Factors influencing the pace of food intake for nursing home residents with dementia: resident characteristics, staff mealtime assistance and environmental stimulation. Nurs open. 2019;6:772–782. doi: 10.1002/nop2.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilmore-Bykovskyi AL, Roberts TJ, Bowers BJ, et al. . Caregiver person-centeredness and behavioral symptoms in nursing home residents with dementia: a timed-event sequential analysis. Gerontologist. 2015;55(supplement):S61–S66. doi: 10.1093/geront/gnu164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilmore-Bykovskyi AL, Rogus-Pulia N. Temporal associations between caregiving approach, behavioral symptoms and observable indicators of aspiration in nursing home residents with dementia. J Nutr Health Aging. 2018;22(3):400–406. doi: 10.1007/s12603-017-0943-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glanz K, Rimer BK, Viswanath K.. Health Behavior and Health Education: Theory, Research, and Practice. 4th ed. John Wiley & Sons; 2008. [Google Scholar]
- 18. Bronfenbrenner U. Ecological systems theory. In: Vasta R, ed. Six Theories of Child Development: Revised Formulations and Current Issues. Jessica Kingsley Publishers; 1992:187–249. [Google Scholar]
- 19. McLaren L, Hawe P. Ecological perspectives in health research. J Epidemiol Community Health. 2005;59(1):6–14. doi: 10.1136/jech.2003.018044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:1–16. doi: 10.1136/bmj.h369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Algase DL, Beck C, Kolanowski A, et al. . Need-driven dementia-compromised behavior: an alternative view of disruptive behavior. Am J Alzheimer’s Dis. 1996;11(6):10–19. doi: 10.1177/153331759601100603 [DOI] [Google Scholar]
- 22. Kovach CR, Noonan PE, Schlidt AM, et al. . A model of consequences of need‐driven, dementia‐ compromised behavior. J Nurs Scholarsh. 2005;37(2):134–140. doi: 10.1111/j.1547-5069.2005.00025_1.x [DOI] [PubMed] [Google Scholar]
- 23. Smith M, Gerdner LA, Hall GR, et al. . History, development, and future of the progressively lowered stress threshold: a conceptual model for dementia care. J Am Geriatr Soc. 2004;52(10):1755–1760. doi: 10.1111/j.1532-5415.2004.52473.x [DOI] [PubMed] [Google Scholar]
- 24. Liu W, Williams KN, Chen Y. Sequential dependencies in solid and fluid intake in nursing home residents with dementia: a multistate model. The Gerontological Society of America (GSA)’s 72nd Annual Scientific Meeting (Virtual); 2020. Late Breaking Poster. [Google Scholar]
- 25. Williams KN, Perkhounkova Y, Herman R, et al. . A communication intervention to reduce resistiveness in dementia care: a cluster randomized controlled trial. Gerontologist. 2016;57(4):707–718. doi: 10.1093/geront/gnw047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu W, Batchelor M, Williams KN. Ease of use, feasibility, and inter-rater reliability of the refined Cue Utilization and Engagement in Dementia (CUED) mealtime video-coding scheme. J Adv Nurs. 2020;76:3609–3622. doi: 10.1111/jan.14548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu W, Kim S. Dyadic interactions and physical and social environment in dementia mealtime care: a systematic review of instruments. Ann N Y Acad Sci. 2021;1505(1), 23–39. doi: 10.1111/nyas.14667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. SAS Institute Inc. SAS 9.4 Language Reference: Concepts. 6th ed. SAS Institute Inc.; 2017. [Google Scholar]
- 29. Sclan SG, Reisberg B. Functional Assessment Staging (FAST) in Alzheimer’s disease: reliability, validity, and ordinality. Int Psychogeriatr. 1992;4(supplement 1):55–69. doi: 10.1017/s1041610292001157 [DOI] [PubMed] [Google Scholar]
- 30. Knoefel FD, Patrick L. Improving outcomes in geriatric rehabilitation: the impact of reducing cumulative illness. Geriatr Today. 2003;6:153–157. [Google Scholar]
- 31. Cohen P, West SG, Aiken LS.. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Psychology Press; 2014. doi: 10.4324/9781410606266 [DOI] [Google Scholar]
- 32. Droogsma E, van Asselt D, De Deyn PP. Weight loss and undernutrition in community-dwelling patients with Alzheimer’s dementia: from population based studies to clinical management. Z Gerontol Geriatr. 2015;48(4):318–324. doi: 10.1007/s00391-015-0891-2 [DOI] [PubMed] [Google Scholar]
- 33. Hadde EK, Chen J. Texture and texture assessment of thickened fluids and texture‐modified food for dysphagia management. J Texture Stud. 2021;52(1):4–15. doi: 10.1111/jtxs.12567 [DOI] [PubMed] [Google Scholar]
- 34. Simmons SF, Keeler E, Zhuo X, et al. . Prevention of unintentional weight loss in nursing home residents: a controlled trial of feeding assistance. J Am Geriatr Soc. 2008;56(8):1466–1473. doi: 10.1111/j.1532-5415.2008.01801.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simmons SF, Patel AV. Nursing home staff delivery of oral liquid nutritional supplements to residents at risk for unintentional weight loss. J Am Geriatr Soc. 2006;54(9):1372–1376. doi: 10.1111/j.1532-5415.2006.00688.x [DOI] [PubMed] [Google Scholar]
- 36. Simmons SF, Osterweil D, Schnelle JF. Improving food intake in nursing home residents with feeding assistance: a staffing analysis. J Gerontol A Biol Sci Med Sci. 2001;56(12):M790– M794. doi: 10.1093/gerona/56.12.m790 [DOI] [PubMed] [Google Scholar]
- 37. Simmons SF, Schnelle JF. Individualized feeding assistance care for nursing home residents: staffing requirements to implement two interventions. J Gerontol A Biol Sci Med Sci. 2004;59(9):M966– M973. doi: 10.1093/gerona/59.9.m966 [DOI] [PubMed] [Google Scholar]
- 38. Whitlatch CJ, Orsulic-Jeras S. Meeting the informational, educational, and psychosocial support needs of persons living with dementia and their family caregivers. Gerontologist. 2018;58(suppl_1):S58–S73. doi: 10.1093/geront/gnx162 [DOI] [PubMed] [Google Scholar]
- 39. Scales K, Zimmerman S, Miller SJ. Evidence-based nonpharmacological practices to address behavioral and psychological symptoms of dementia. Gerontologist. 2018;58(suppl_1):S88–S102. doi: 10.1093/geront/gnx167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gilster SD, Boltz M, Dalessandro JL. Long-term care workforce issues: practice principles for quality dementia care. Gerontologist. 2018;58(suppl_1):S103–S113. doi: 10.1093/geront/gnx174 [DOI] [PubMed] [Google Scholar]
- 41. Calkins MP. From research to application: supportive and therapeutic environments for people living with dementia. Gerontologist. 2018;58(suppl_1):S114–S128. doi: 10.1093/geront/gnx146 [DOI] [PubMed] [Google Scholar]
- 42. Liu W, Jao YL, Williams KN. The association of eating performance and environmental stimulation among older adults with dementia in nursing homes: a secondary analysis. Int J Nurs Stud. 2017;71:70–79. doi: 10.1016/j.ijnurstu.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.