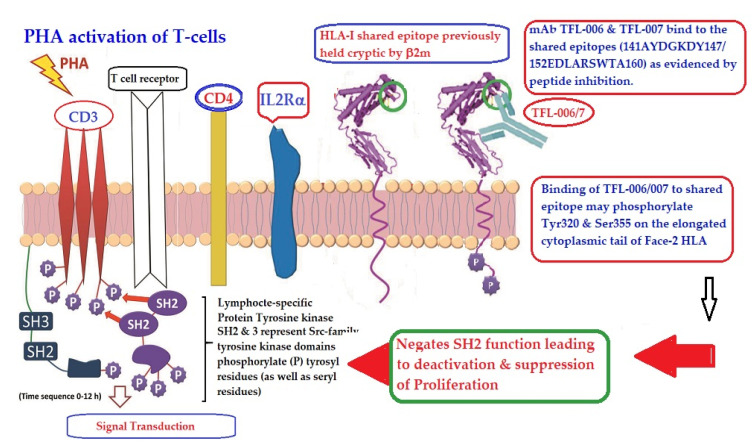
Figure 13.
An illustration of the possible mechanism of suppression of PHA activated T-cells mediated by mAbs directed against shared epitopes exposed in the Face-2 of almost all alleles of HLA-I. The figure shows CD3/T cell receptor (TCR)/CD4 on the bi-layered lipid membrane on the T-cells upon activation (Yellow complex arrow). Phosphorylation of the cytoplasmic domain of CD3 is induced by Lymphocyte-specific protein tyrosine kinase (LCK). SH represents Scr-family LCK. They phosphorylate the tyrosyl (even seryl) domain of the cytoplasmic tail of CD3. This event leads to activation of transcription factors and transcription cell-surface molecules such as IL2Rα and Face-2. Importantly, the HLA-I open conformers devoid of β2m (Face-2) expose unique epitopes shared by almost all HLA-I alleles. It is the site recognized by TFL-006 and TFL-007. It is postulated that the binding of these specific mAbs to the epitopes on the Face-2 may initiate phosphorylation of the elongated cytoplasmic tail. The elongated cytoplasmic tail results in the exposure of cryptic tyrosyl residue at position 320 [137,138,139] and serine at position 35 [140]. This leads to transduction that initiates dephosphorylation of the cytoplasmic domain of CD3, resulting in suppression of activation of T-cells. Evidently, the T-cell activating CD3-phosphorylation can be reversed.