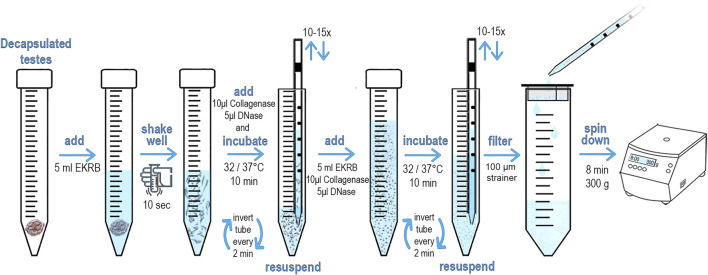
FIGURE 1.
Schematic diagram and protocol of preparation of testicular single cell suspension. The male mouse was euthanized. An incision was them made under scrotum, testes were removed, dissected and transferred to a Petri dish. Using scissors, the tunica albuginea was opened to release the seminiferous tubules (STs). STs were immediately placed into 15 ml conical tube containing 5 ml of freshly made pre-warmed EKRB. The tissue was disrupted by vigorously shaking by hand for at least 10 s 10 μL Collagenase (50 mg/ml) and 5 μL of DNase (10.000U) was then immediately added and incubated at 32°C/37°C (according to age of the male) for 10 min. The samples were inverted every 2 minutes to keep the tubules in suspension. STs are then disrupted by slowly pipetting through serological pipet. Repeat 10–15 times until STs are fragmented. An additional 5 ml of EKRB, 10 μL Collagenase (50 mg/ml) and 5 μL of DNase (10.000U) is added and incubated for another 10 min at the desired temperature. The samples were inverted every 2 minutes to keep the tubules in suspension. The remaining ST fragments are disrupted by slowly pipetting through a serological pipet. Pipet up and down at least 10–15 times until the suspension appears opaque and the ST fragments are no longer visible. Transfer the cell suspension dropwise with serological pipet into a new conical tube that is equipped with an inserted 100 μm strainer to obtain a ST single cell suspension. Centrifuge the cell suspension for 8 min at 300 g to pellet the cells. Discard the supernatant and add 200 μL of pre-warmed EKRB. Gently re-suspend pelleted cells by tapping on the tube bottom. Add the conjugated antibody and incubate at 32°C/37°C in a dark place.