Abstract
Infectious diseases caused by bacteria and fungi are threatening human health all over the world. It is an increasingly serious problem that the efficacies of some antibacterial and antifungal agents have been weakened by the drug resistance of some bacteria and fungi, which makes a great need for new antibiotics. Sesquiterpenoids, with abundant structural skeleton types and a wide range of bioactivities, are considered as good candidates to be antibacterial and antifungal agents. In the past decades, many sesquiterpenoids were isolated from plants and fungi that exhibited good antibacterial and antifungal activities. In this review, the names, source, structures, antibacterial and antifungal degrees, and mechanisms of sesquiterpenoids with antibacterial and antifungal activity from 2012 to 2022 are summarized, and the structure-activity relationship of these sesquiterpenoids against bacteria and fungi is also discussed.
Keywords: sesquiterpenoids, chemical structures, antibacterial activity, antifungal activity, mechanism, structure–activity relationship
1. Introduction
The infections caused by drug-resistant bacteria and drug-resistant fungi are increasing across the world, and the threat of untreatable infections has been looming since the 21st century [1]. About 4.95 million people died from diseases related to antibiotic-resistant bacteria in 2019, and 1.27 million deaths were directly caused by antibiotic-resistant bacteria, which indicated that drug-resistant infections killed more people than HIV/AIDS (864,000 deaths) or malaria (643,000 deaths) [2]. Fungi also led to life-threatening systemic infections, with a mortality of over 1.6 million, which is three times more than malaria, resulting in the widespread use of antifungal agents [3]. The efficacy of the limited systemic antifungal drugs was counteracted by fungal attributes and host- and drug-related factors. Furthermore, some fungal pathogens showed notable rates of antifungal resistance, including Candida, Aspergillus, Cryptococcus, and Pneumocystis [4]. Therefore, it is a challenge that antibiotic resistance is not easy to overcome, requiring the development of newer antibacterial and antifungal drugs [3].
Natural products, which have rich resources and great bioactivities, play an important role in the discovery of new drugs [5]. A total of 60% of the small molecule drugs marketed from 1981 to 2019 arose from natural products or synthetic molecules based on natural product pharmacophores [6]. Sesquiterpenoids are the most abundant natural products, with various activities and excellent prospects in drug development. For example, qinghaosu (artemisinin), a sesquiterpenoid lactone from Artemisia annua discovered by Tu Youyou, has already reached the market as an antimalarial drug [7,8]. Santonin, a sesquiterpenoid compound, has been marketed as an anthelmintic and used for a long time against ascaris infection with a remarkable curative effect which exhibited certain inhibitory effects on bacteria and fungi [9,10]. There are some other sesquiterpenoids compounds, such as parthenolide, alantolactone, bilobalide, coriaria lactone, and cycloeudesmol, that have already been commercialized [11,12,13,14,15]. Aiming to look for better antimicrobial leads, the names, structures, source, antibacterial and antifungal degree, and mechanisms of natural sesquiterpenoids with antibacterial activity from 2012 to 2022 are systematically and completely summarized in this review (Tables S1 and S2). The structure–activity relationship of sesquiterpenoids with significant antibacterial and antifungal activity is discussed as well. This review will provide support for the use of sesquiterpenoids as potential antibacterial agents in the future.
2. Sesquiterpenoids Types
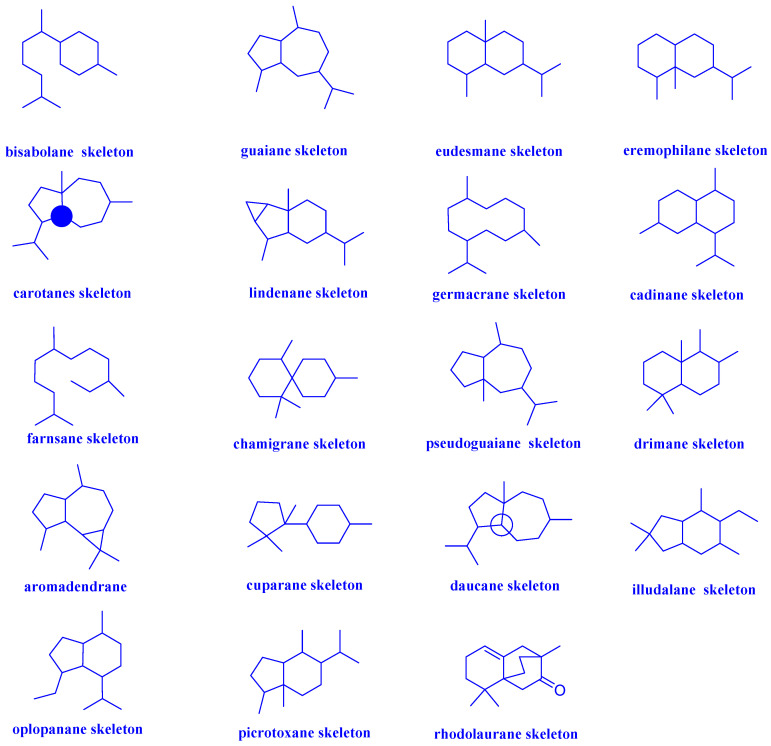
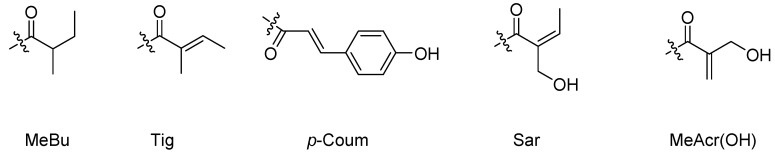
Sesquiterpenoids, C15 compounds composed of three isoprene units, are one family of structurally diverse natural products [16]. Today, thousands of sesquiterpenoids have been discovered with more than 100 skeleton types. Regarding their carbon skeletons, sesquiterpenoids with antibacterial and antifungal activity mainly include bisabolane, guaiane, eudesmane, eremophilane, carotane, lindenane, germacrane, cadinane, farnsane, chamigrane, pseudoguaiane, drimane, aromadendrane, cuparane, daucane, illudalane, oplopanane, picrotoxane, rhodolaurane and other types, which were showed in Figure 1. The groups used shorthands mentioned in compounds structure were demonstrated in Figure 2.
Figure 1.
Different types of sesquiterpenoid skeletons.
Figure 2.
Groups in shorthand.
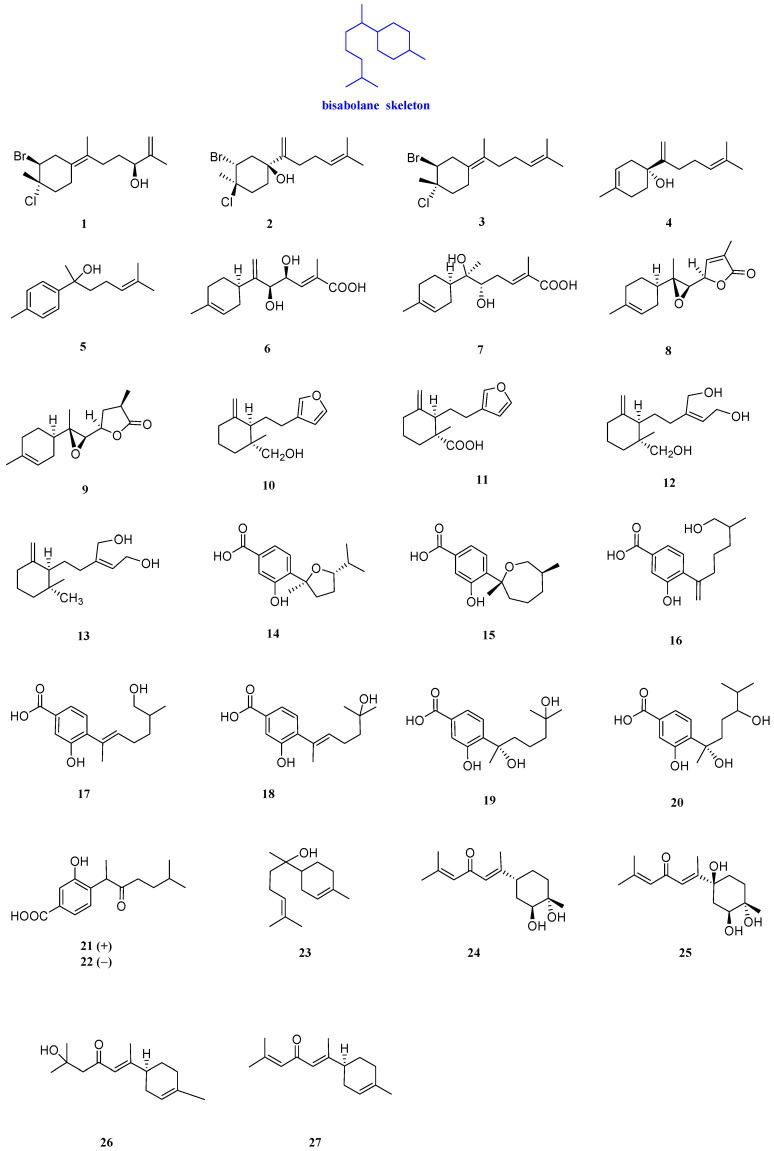
2.1. Bisabolanes
Laurecomposin A, laurecomposin B, preintricatol, helianthol B, and gossonorol (1–5, Figure 3) were isolated from the red alga Laurencia tristicha. The complete 1H and 13C NMR assignments of compound 1 were made by a combination of 1H, 13C, DEPT, 1He1H COSY, HSQC, HMBC, and ROESY experiments and the absolute configuration was established by the modified Mosher’s method. It was confirmed that compound 1 had activity against Staphylococcus aureus (S. aureus) and Candida albicans (C. albicans) SC5314 with MIC values of 26.8 and 16 µg/mL, respectively. Compound 1 also had an obvious inhibitory effect on Microsporum gypseum (M. gypseum), and the MIC value was 4.0 µg/mL. In the antifungal and antibacterial assays, compound 2 exhibited significant inhibitory activities against M. gypseum (Cmccfmza) and moderate activities towards S. aureus, with MIC values of 8 and 15.4 μg/mL, respectively. Compound 3 had inhibitory activity against C. albicans SC5314, S. aureus, and M. gypseum, with MIC values of 32, 13.6, and 8 μg/mL. In addition, compounds 4 and 5 had inhibitory activity against S. aureus and M. gypseum, with MIC values of 4−54 μg/mL [17].
Figure 3.
Structures of bisabolanes-type sesquiterpenoids 1–27.
Six sesquiterpenoids with antibacterial activity were isolated from a basidiomycete collected in Mount Elgon Natural Reserve, named elgonene C, elgonene D, elgonene G, elgonene H, elgonene I, elgonene J, elgonene K, and elgonene L (6–13, Figure 3). To determine the stereochemistry of elgonene J, elgonene K, and elgonene L, CD spectra of these three compounds were measured. Compounds 6–13 exhibited an inhibitory effect on M. hiemalis DSM 2656, with MIC values of 25 to 100 µg/mL. Compounds 6, 8/9, and 13 had weak inhibitory activity against S. aureus DSM 346 with a MIC value of 100 μg/mL. Compounds 8–11 and 13 (8/9 were tested as an inseparable mixture) showed weak antimicrobial activity against Bacillus subtilis (B. subtilis) DSM 10, with MIC values of 100, 100, 75, 75, and 100 μg/mL, respectively. Compounds 8/9 and 13 demonstrated weak activities against Micrococcus luteus (M. luteus) DSM 1790 (same MIC value of 100 μg/mL) and moderate activity against M. hiemalis DSM 2656 (MIC values of 100 and 25 μg/mL, respectively). No activity against Gram-negative bacteria or yeast was observed. Compounds 6–9 and 11–13 showed antibacterial activity against M. hiemalis DSM 2656, with MIC values of 100, 50, 100, 50, 50, 25, and 50 μg/mL [18].
There were seven bisabolane-type sesquiterpenoids with antibacterial activity from the leaves of a Thai mangrove Xylocarpus moluccensis, named (7S,10S)-7,10-epoxysydonic acid, (7R,11S)-7,12-epoxysydonic acid, 7-deoxy-7,14-didehydro-12-hydroxysydonic acid, (E)-7- deoxy-7, 8-didehydro-12-hydroxysydonic acid, engyodontiumone I, (+)-hydroxysydonic acid, and (−)-(7S)-10-hydroxysydonic acid (14–20, Figure 3). The potent inhibitory activitiy of compounds 14–20, for S. aureus ATCC 25923, was evaluated by liquid phase inhibition in 96-well microplates, and the IC50 values were determined to be at 31.5–41.9 µM [19].
(+)-Phomoterpene A and (−)-phomoterpene A (21 and 22, Figure 3), isolated from the endophytic fungus Phomopsis prunorum (F4-3), showed inhibitory activity against X. citri pv. Phaseoli var. fuscans, with MIC values of 31.2 and 62.4 µg/mL, respectively. Additionally, both of the compounds exhibited inhibitory against the plant pathogen Pseudomonas syringae pv (P. syringae pv). Lachrymans had the same MIC value of 15.6 µg/mL [20].
α-Bisabolol (23, Figure 3) is a low toxic monocyclic sesquiterpenoid alcohol from Vanillosmopsis arborea Barker. The IC50s of compound 23 against CA INCQS 40006, CK INCQS 40095, and CT INCQS 40042, different strains of Candida, were 8.92, 18.15, and 18.26 µM, respectively [21]. (E)-(2S,3S,6R)-Atlantone-2,3-diol, (E)-(2S,3S,6S)-atlantone-2,3,6-triol, atlantolone, and (E)-α-atlantone (24–27, Figure 3) were isolated from Cedrus deodara Loud. Compound 24 had a weak inhibitory effect on Aspergillus sydowii (A. sydowii) and Aspergillus parasiticus (A. parasiticus), with MIC values of 6400 and 3200 µg/mL, respectively. Compound 25 was active against Trichophyton rubrum (T. rubrum), with a MIC value of 125 µg/mL, while compounds 26 and 27 exhibited an inhibitory effect on Aspergillus niger (A. niger), A. sydowii, A. parasiticus, A. ochraceous, and A. flavus, with MIC values from 200 to 6400 µg/mL [22].
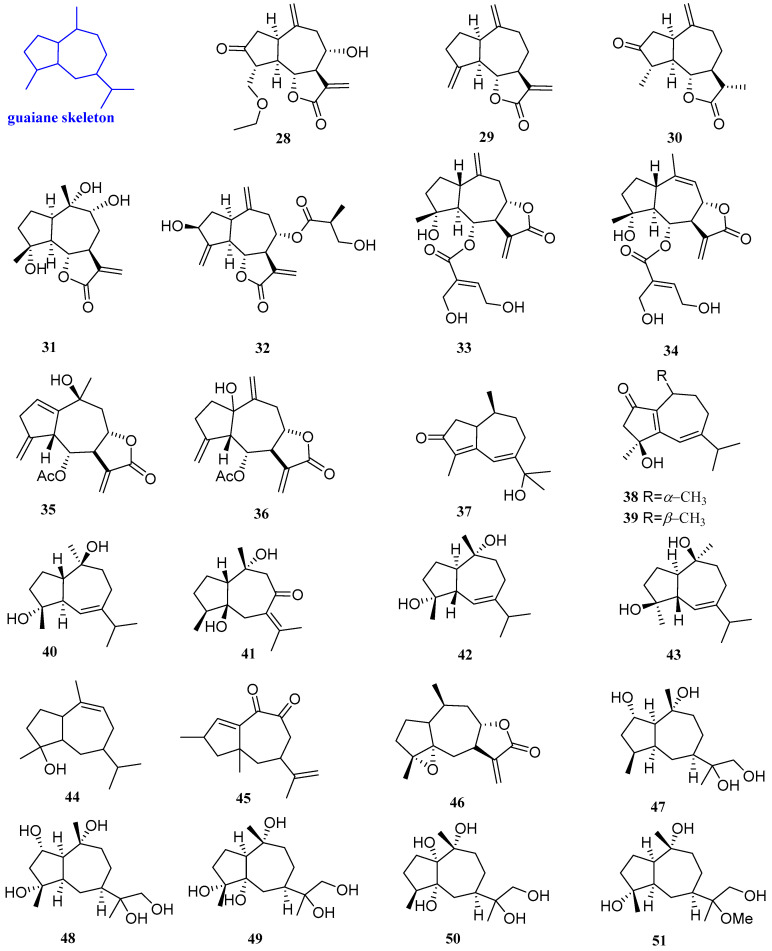
2.2. Guaianes
Three new sesquiterpenoids were isolated from Artemisia vestita, including artemivestinolide G, dehydrocostuslactone, and dihydroestafiatone (28−30, Figure 4). The antifungal test revealed that 29 had a good inhibitory effect on Fusarium oxysporum (F. oxysporum), with a MIC value of 256 mg/L. Compounds 28 and 30 had a certain antibacterial effect on Botrytis cinerea (B. cinerea), with a MIC value of 256 mg/L [23].
Figure 4.
Structures of guaiane-type sesquiterpenoids 28–51.
4α,9α,10α-Trihydroxyguaia-11(13)en-12,6α-olide (31, Figure 4) was collected from the leaves of the Saudi medicinal plant Anvillea garcinii. Compound 31 had antibacterial activity against C. albicans, C. parapsilosis, S. aureus, B. licheniformis, and E. fergusonii, with MIC values of 0.21, 0.25, 2.3, 2.3, and 5.7 µg/mL, respectively [24]. 8-O-[3’-Hydroxy-2’-methylpropionate] (32, Figure 4), extracted from the aerial parts of Centaurea rhizantha, had moderate antibacterial activity against S. aureus, and the MIC/MBC value was 500 µg/mL. [25].
6α-[4’,5’-Dihydroxytigloyloxy]-inuviscolide and 6α-[4’,5’-dihydroxytigloyloxy]-isoinuviscolide (33 and 34, Figure 4) were extracted from Schkuhria pinnata (Lam.) Kuntze ex thell. A micro broth dilution method was used to test the antibacterial activity of compounds 33 and 34. The results showed that compounds 33 and 34 both had antibacterial activity against Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), Enterobacter faecalis (E. faecalis), and S. aureus, and the MIC values were 125, 46.88, 125, and 62.5 µg/mL [26].
6-Acetoxy-10-β-hydroguaiantrienolide and 6-acetoxy-1α-hydroguaiantrienolide (35 and 36, Figure 4) were isolated from Cotula cinerea. Compounds 35 and 36 had antibacterial activity against some strains of Enterobacter faecalis, EF-91804, EF-91823, EF-165, EF-91705, and ATCC29212, and the MICs were 150, 300, 300, 300, and 300µg/mL, respectively. [27]. Sootepdienone, jambolanins E, jambolanins F, and guaianediol (37–40, Figure 4) were isolated from the seeds of Eugenia jambolana fruit and exhibited inhibitory activity against S. aureus, with a diameter of the inhibitory zone of 9, 10, 10, and 9 mm, respectively [28].
Wenyujinin Q (41, Figure 4), isolated from a traditional Chinese medicine, Curcuma wenyujin dreg, had strong broad-spectrum antifungal activity against nine pathogenic fungi, including Alternaria brassicicola (A. brassicicola), P. parasitica var. nicotianae, C. capsici, B. oryzae, D. medusaea Nitschke, C. paradoxa Moreau, E. turcicum, P. theae, and A. citri (the MICs being 50, 100, 50, 50, 100, 50, 25, 25, and 100 µg/mL, respectively) [29]. 4α,10α-Dihydroxy-5β-H-guaja-6-ene (42, Figure 4) was isolated from Cassia buds. 42 had antibacterial activity against C. albicans and S. aureus, with inhibitory zones of 9 and 7.5 mm, respectively [30].
4β, 10β-Dihydroxy-1αH, 5βH-guaia-6-ene (43, Figure 4), obtained from the rhizome of Alisma orientale, could inhibit the activity of B. subtilis and possessed a MIC value of 50 µg/mL [31]. Two sesquiterpenoids named guai-9-en-4β-ol and 14,15-dinorguai-1,11-dien-9,10-dione (44 and 45, Figure 4) were isolated from the stem of Syringa pinnatifolia Hemsl. var. alashanensis. Compound 44 had strong inhibition against E. coli, S. aureus, B. coagulas, Proteus vulgaris (P. vulgaris), P. digitatum, F. oxysporum, and A. niger, with inhibitory zones of 11.02, 13.41, 15.34, 9.67, 12.56, 11.64, and 13.20 mm, respectively. Compound 45 had good inhibitory activity against E. coli, S. aureus, B. coagulas, P. vulgaris, P. digitatum, F. oxysporum, and A. niger, with inhibitory zones of 15.34, 9.45, 12.01, 14.96, 12.34, 15.32, and 11.53 mm, respectively [32].
4α,5α-Epoxy-10α,14H-1-epi-inuviscolide (46, Figure 4) was isolated from Carpesium macrocephalum. The antibacterial experiments showed that 46 had certain inhibitory effects against C. albicans and the yeast-to-hyphae morphogenetic transition, with IC50 values of 38 and 106.5 µg/mL [33]. Five sesquiterpenoids were isolated from the Endophytic Fungus Xylaria sp. YM 311647 of Azadirachta indica A. Juss., identified as (1S,2S,4S,5S,7R,10R)-Guaiane-2,10,11,12-tetraol, (1S,2S,4R,5R,7R,10R)-Guaiane-2,4,10,11,12-pentaol, (1S,4R,5S,7R,10R)-Guaiane-4,5,10,11,12-pentaol, (1R,4S,5R,7R,10R)-Guaiane-1,5,10,11,12-pentaol, and (1R,4R,5R,7R,10R)-11-Methoxyguaiane-4,10,12-triol, respectively (47–51, Figure 4). The antifungal activity of compounds 47–51 was evaluated by the micro broth dilution method, which indicated that compounds 47–51 exhibited moderate or weak antifungal activities against C. albicans, Pyricularia oryzae (P. oryzae), and Hormodendrum compactum (H. compactum), with MIC values in the range of 32–256 μg/mL. Compounds 47–51 had antibacterial activity against A. niger, with MIC values of 256, 64, 256, and 256 µg/mL [34].
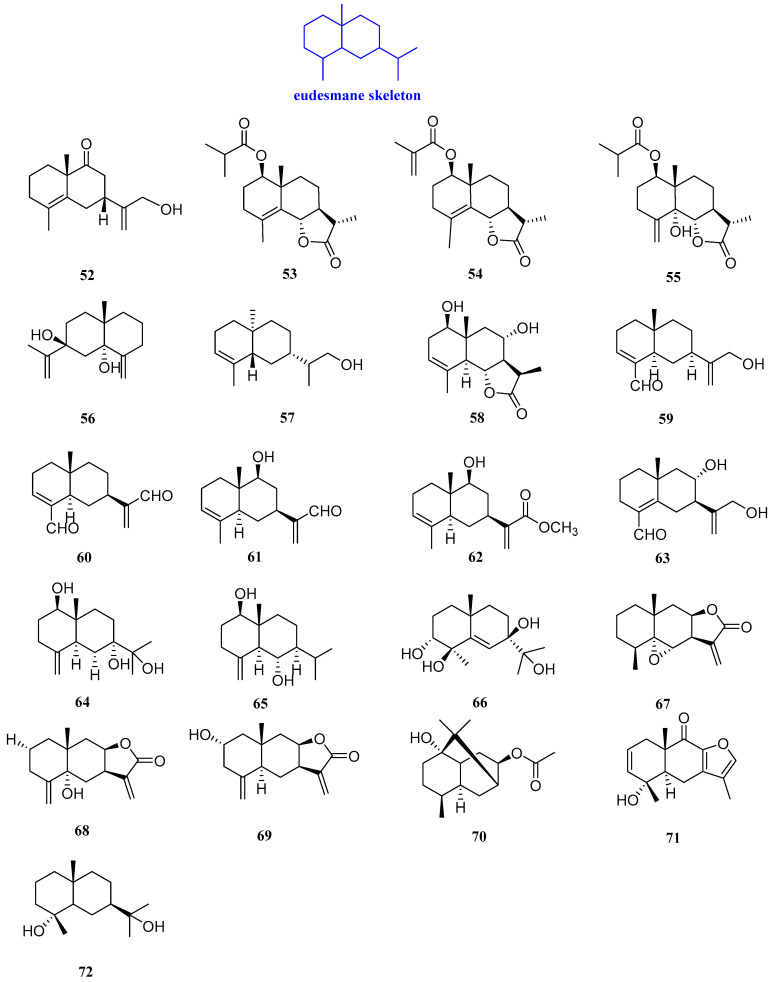
2.3. Eudesmanes
Sutchuenin J (52, Figure 5) is a eudesmane-type sesquiterpenoid extracted from the EtOAc soluble fraction of the ethanolic extract of the stems and roots of Thuja sutchuenensis. Compound 52 displayed good antibacterial activities against Bacillus cereus (B. cereus) (ATCC 10876) and Staphylococcus epidermidis (S. epidermidis) (ATCC 12228), with the same MIC value of 25 µg/mL [35].
Figure 5.
Structures of eudesmane-type sesquiterpenoids 52–72.
Artemivestinolides D–F (53–55, Figure 5) were isolated from Artemisia vestita, Compound 53 had a good inhibitory effect on Pyricularia oryzae, with a MIC value of 128 mg/L. Compounds 53 and 54 against B. cinerea, with the same MIC value of 256 mg/L. For F. oxysporum, compound 55 displayed antifungal activity, with a MIC value of 256 mg/L [23].
Eudesma-4(15),11-diene-5,7-diol (56, Figure 5) was extracted from Laurencia obtusa Lamouroux with antibacterial activity. Compound 56 possessed antifungal activity against C. albicans and Candida tropicalis (C. tropicalis), with MIC values of 8.27 and 10.13 µM, respectively. The antifungal activity of compound 56 was higher than amphotericin B (MICs = 4.63 and 5.27 µM, respectively) [36].
Eutyscoparin G (57, Figure 5) was isolated from the ethyl acetate extract of the endophytic fungus Eutypella scoparia SCBG-8. It was found that compound 57 could inhibit S. aureus and methicillin-resistant S. aureus with the same MIC value of 6.3 µg/mL [37]. 1R,8S-dihydroxy-11R,13-dihydrobalchanin (58, Figure 5) was extracted from the CH2Cl2 extract of Artemisia Sieberi. The agar diffusion technique was followed and the inhibition zone of 58 against B. subtilis, S. aureus, E. coli, F. solani, P. aeruginosa, C. tropicalis, and F. solani was 6–8 mm, which was more effective than thiophenicol, the positive control which was a broad-spectrum antibacterial antibiotic [38].
The antibacterial activities of (4αβ,7β,8αβ)-3,4,4α,5,6,7,8,8α-octahydro-7-[1-(hydroxymethyl)ethenyl]-4α-methylnaphthalene-1-car boxaldehyde, 12,15-dioxo-α-selinen, (5S,7S,9S,10S)-(+)-9-hydroxy-selina-3,11-dien-12-al, (5S,7S,9S,10S)-(+)-9-hydroxy-eudesma-3,11(13)-dien-12-methyl ester, and (7S,8R,10S)-(+)-8,12-dihydroxy-selina-4,11-dien-14-al (59–63, Figure 5), isolated from Chinese agarwood, were measured by inhibition zone diameters. All these compounds could inhibit S.aureus, with inhibitory zones of 9.12, 20.02, 12.90, 14.20, and 8.10 mm, respectively. Compounds 59–63 had antibacterial activity against Ralstonia solanacearum (R. solanacearum), with inhibitory zones of 8.98, 11.02, 18.20, and 10.15 mm, respectively, while 63 had no activity against R. solanacearum [39].
4(15)-Eudesmene-1β,7,11-triol, 1β,6α-dihydroxyeudesm-4(15)-ene, and cinnamosim B (64–66, Figure 5) are three eudesmane-type sesquiterpenoids isolated from Cassia buds which have antimicrobial activity against C. albicans, S. aureus, and E. coli. Compounds 64–66 selectively inhibited the proliferation of C. albicans, with inhibitory zones of 9, 11, and 10 mm, respectively. Compounds 65 and 66 could also inhibit the proliferation of S. aureus, with inhibitory zones of 11 and 9 mm, respectively. Compound 64 only showed inhibitory effects on E. coli, with an inhibitory zone of 8.5 mm [30].
Three germacrane-type sesquiterpenoids were extracted and isolated from the whole plant of Carpesium macrocephalum, named 5α-epoxyalantolactone, telekin, and ivalin (67–69, Figure 5). Compounds 68 and 69 inhibited biofilm formation of C. albicans, with IC50 values ranging from 15.4 to 36.0 μg/mL, and compound 67 inhibited the yeast-to-hyphae morphogenetic transition, with an IC50 value of 118.4 μg/mL [33].
8-Acetoxyl-pathchouli alcohol (70, Figure 5) was isolated from the roots of Valeriana jatamansi Jones, and its antibacterial activity was identified by the micro broth dilution method. The antibacterial assays revealed that compound 70 had a certain inhibitory effect on S. aureus and P. aeruginosa, with MIC values of 128 and 64 µg/mL, respectively [40].
A sesquiterpenoid lactone named chlojaponol B (71, Figure 5) was isolated from Chloranthus japonicus. Compound 71 displayed a certain antibacterial activity against B. cinerea and S. sclerotiorum, with inhibitory rates of 34.62% and 13.04% at the concentration of 50 μg/mL [41].
Cryptomeridiol (72, Figure 5), isolated from the seeds of Eugenia jambolana fruit, exhibited inhibitory activity against S. aureus, with a diameter of the inhibitory zone of 8 mm [28].
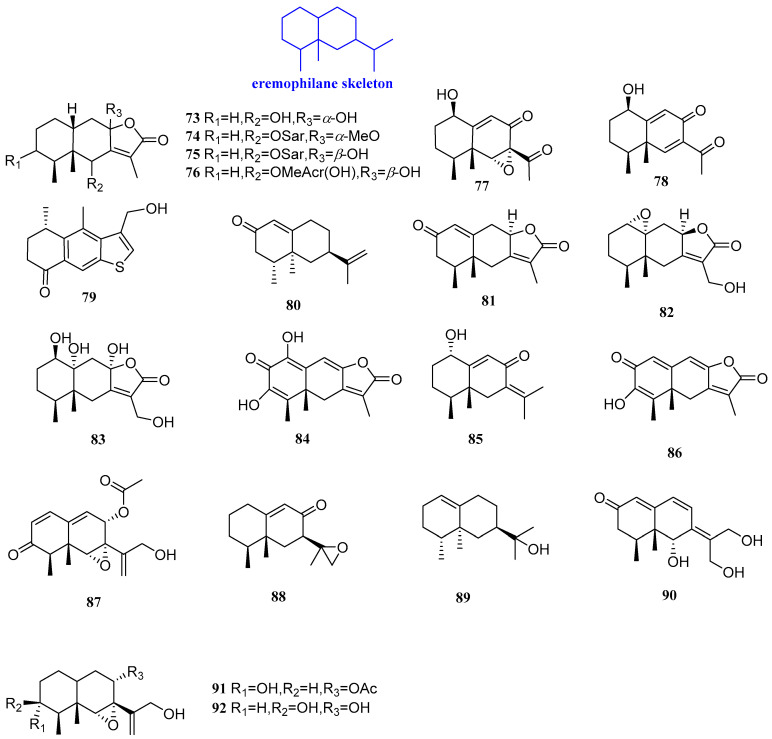
2.4. Eremophilanes
Six sesquiterpenoids were isolated from Ligularia sagitta, named 1β,10β-epoxy-6β,8α-dihydroxyeremophil-7(11)-en-8β(12)-olide, sagittacin C, sagittacin D, 6β-(2’-hydroxymethylacryloyloxy)-1β,10β-epoxy-8β-hydroxyeremophila-7(11)-en-8α(12)-olide, sagittacin E, and 1β-hydroxy-6,9-dien-8-oxoeremophil-11-nor-11-ketone (73−78, Figure 6). It was confirmed that compounds 73−76 could inhibit B. cereus, S. aureus, B. subtilis, E. coli, and Erwinia carotovora (E. carotovora), with MIC values ranging from 7.25 to 125 µg/mL. Compound 78 exhibited moderate activity against E. coli and E. carotovora, with MIC values of 31.25 µg/mL and 62.5 µg/mL, respectively, and compound 78 showed only moderate inhibitory effects against E. coli, with a MIC value of 31.25 µg/mL [42].
Figure 6.
Structures of eremophilane-type sesquiterpenoids 73–92.
Leptosphin A (79, Figure 6) was purified from the solid fermentation cultures of the endophytic fungus Leptosphaeria sp. XL026, isolated from the leaves of Panax notoginseng. The antibacterial and antifungal activities of compound 79 were tested. The results showed that compound 79 had antifungal activity against Fusarium graminearum (F. graminearum), S. sclerotiorum, V. dahliae Kleb, B. carbonum Wilson, P. parasitica Dastur, A. alternata (Fries) Keissler, and B. cinerea Pers, with the MIC value range of 25–100 µg/mL. Furthermore, compound 79 also showed medium antibacterial activity against Micrococcus lysodeikticus (M. lysodeikticus), B. cereus, S. aureus, S. typhimurium, and E. aerogenes, with MIC values of 50, 25, 100, 100, and 50 µg/mL, respectively [43]. Nootkatone (80, Figure 6) exists in grapefruit and has a variety of pharmacological effects. Researchers found that 80 had an inhibitory effect at the concentrations of 1 and 0.5 mM, respectively, for L. monocytogenes and C. diphtheriae [44].
Xylareremophil and eremophilane mairetolides B and G (81–83, Figure 6) were isolated from the endophytic fungus Xylaria sp. GDG-102, cultured from Sophora tonkinensis. For P. vulgaris and Micrococcus luteum (M. luteum), 81 displayed moderate activity, with the same MIC value of 25 µg/mL, and the MIC values of 83 were 25 μg/mL and 50 μg/mL, respectively. Compound 81 was found to be active against M. luteum with a MIC value of 50 μg/mL. Compounds 81–83 showed inhibitory effects on both B. subtilis and M. lysodeikticus, with a same MIC value of 100 µg/mL [45].
Rhizoperemophilane K, 1α-hydroxyhydroisofukinon, and 2-oxo-3-hydroxy-eremophila-1 (10),3,7 (11),8-tetraen-8,12-olide (84–86, Figure 6) were isolated from Rhizopycnis vagum. Compounds 84–86 had an inhibitory effect on four kinds of bacteria, including P. lachrymans, R. solanacearum, S. haemolyticus, and X. vesicatoria, with MIC values of 32–128 µg/mL [46]. 8α-Acetoxyphomadecalin C (87, Figure 6) was isolated from the endophyte Microdiplodia sp. WGHS5 and was evaluated for antifungal and antibacterial activity. The results revealed that 87 had equivalently effective antifungal activity against B. cinerea and F. graminearum at 100 μg/mL. [47].
7αH-9(10)-Ene-11,12-epoxy-8-oxoeremophilane and valerianol (88 and 89, Figure 6), were isolated from Chinese agarwood originating from Aquilaria sinensis (Lour.) Gilg. Compound 88 could inhibit S. aureus and R. solanacearum, with an inhibitory area of 12.35 and 16.90 mm, and compound 89 could inhibit S. aureus and R. solanacearum, with an inhibitory area of 10.10 and 8.86 mm [48].
Phomadecalin F, 8α-monoacetoxyphomadecalin D, and 3-epi-phomadecalin D (90−92, Figure 6) were isolated from the endophyte Microdiplodia sp. TT-12. Compounds 91 and 92 showed moderate antimicrobial activity against P. aeruginosa ATCC 15442 and S. aureus NBRC 13276, with an inhibitory area of 10–13 mm. Compound 90 could inhibit P. aeruginosa ATCC 15442, with an inhibitory zone of 8 mm [49].
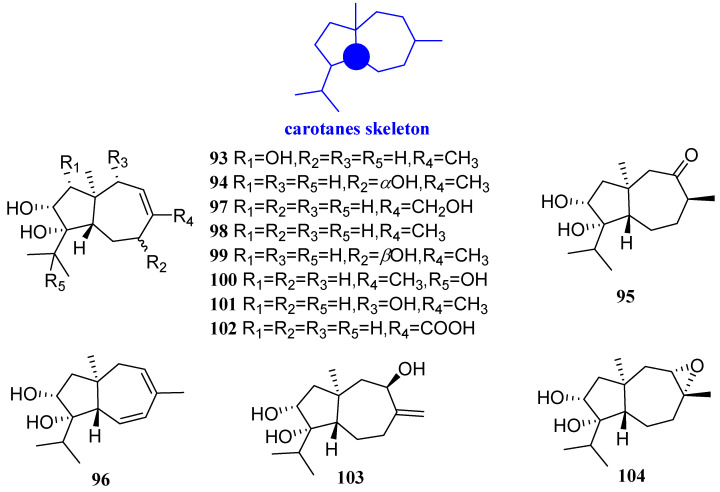
2.5. Carotanes
Trichocarotins I–M, CAF-603, 7β-hydroxy CAF-603, trichocarotins E–H, trichocarane A (93–104, Figure 7) were found in the endophytic fungus Trichoderma virens QA-8 in the inner root tissue of mugwort leaves. The antibacterial activities of these compounds were assayed against human pathogens E. coli EMBLC-1 and M. luteus QDIO-3. Each of the compounds showed an inhibitory activity against E. coli, with the MIC values ranging from 0.5 to 32 µg/mL, and the activity of compounds 95–100 and 103 against E. coli, with the same MIC value of 0.5 µg/mL, which was as active as that of the positive control (chloramphenicol, MIC = 0.5 µg/mL). In addition, compounds 94, 97–99, 103, and 104 showed inhibitory activity against M. luteus, with the MIC values ranging from 0.5 to 32 µg/mL. Compound 99 showed potent activity against M. luteus, with MIC values of 0.5 µg/mL, which was stronger than that of chloramphenicol (MIC = 1 µg/mL) [50].
Figure 7.
Structures of carotane-type sesquiterpenoids 93–104.
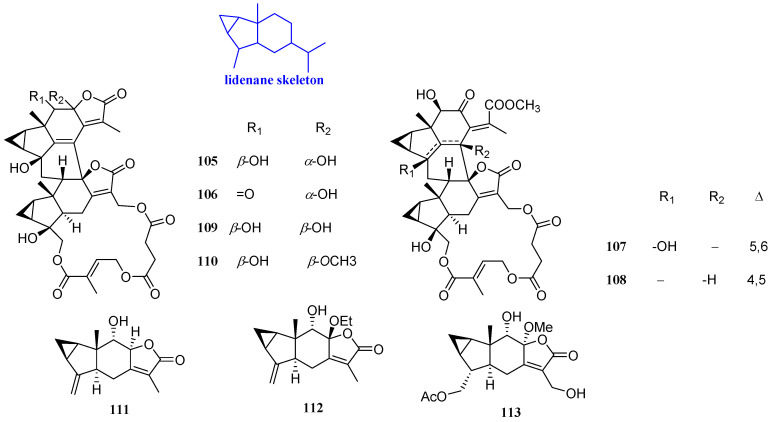
2.6. Lindenanes
Six sesquiterpenoids, named henriol A, spicachlorantin A, chloramultilide A, shizukaol B, tianmushanol, and 8-O-methyltianmushanol (105–110, Figure 8), with antibacterial effects were isolated from the roots of Chloranthus angustifolius. Their antifungal activities were studied by microdilution method. Compounds 105–110 could inhibit the activity of C. albicans, and the MIC values were 4 to 8 µg/mL [51].
Figure 8.
Structures of lindenane-type sesquiterpenoids 105–113.
Three sesquiterpenoid lactones, named chlojaponilactones G-I (111–113, Figure 8), were isolated from Chloranthus japonicus. Compounds 111–113 displayed a certain antibacterial activity against B. cinerea and S. sclerotiorum, with inhibitory rates of 7.69% to 82.61% at the concentration of 50 μg/mL [41].
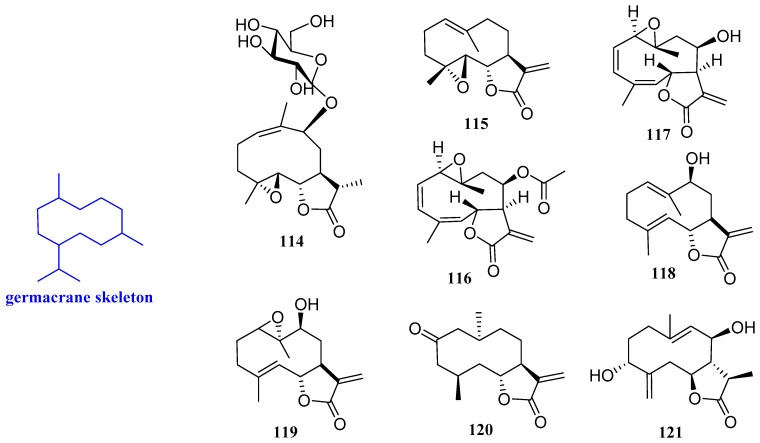
2.7. Germacranes
9β-Hydroxyparthenolide-9-O-β-D-glucopyranoside (114, Figure 9) was obtained from the leaves of the Saudi medicinal plant Anvillea garcinii. Compound 114 showed an inhibitory activity against human pathogenic fungi, which was about 80% at 50 μg/mL against C. albicans and C. parapsilosis, with MIC values of 0.26 μg/mL and 0.31 μg/mL, respectively. In addition, compound 114 inhibited S. aureus, B. licheniformis, and E. fergusonii, with MIC values of 3.4, 3.1, and 6.3 µg/mL, respectively [24].
Figure 9.
Structures of germacrane-type sesquiterpenoids 114–121.
Parthenolide (115, Figure 9) was isolated from Asteraceae and Magnoliaceae and is effective against various plant-pathogenic pathogens. The antibacterial assays revealed that compound 115 had a good inhibitory effect on Erwinia amylovora (E. amylovora) and Corynebacterium fascians (C. fascians), with the same MIC value of 20 mg/L. In addition, parthenolide is also effective against V. mali, A. brassicicola, and P. piricola, with EC50 values of 5, 2, and 5 mg/L, respectively [52].
Incomptine A and incompetine B (116 and 117, Figure 9) showed antibacterial activity against Vibrio cholerae (V. cholerae), with MIC values of 0.15 mg/mL and 0.05 mg/mL, respectively. The antibacterial activity of compounds 116 and 117 was better than chloramphenicol, which was used as positive control. This result suggested that 116 and 117 may be potential antibiotics for chloramphenicol-resistant bacteria, especially for V. cholerae [53].
Haagenolide and 1,10-epoxyhaagenolide (118 and 119, Figure 9) are two germacrane-type sesquiterpenoids isolated from the dichloromethane extract obtained from the aerial parts of Cotula cinerea. The absolute configuration was assigned by applying the advanced Mosher’s method to haagenolide and by X-ray diffraction analysis to 1,10-epoxyhaagenolide. E. faecalis EF-91804, E. faecalis EF-91823, E. faecalis EF-165, and E. faecalis EF-91705 were four clinical bacteria isolated from E. faecalis and used to evaluate the antimicrobial activity of compounds 118 and 119. The results indicated that compound 118 could act against all mentioned E. faecalis above, with the same MIC value of 300 µg/mL. Compound 119 inhibited EF-91804, EF-91823, and EF-165, with the same MIC value of 300 µg/mL, while it only inhibited EF-91705 with a MIC value of 150 µg/mL. Therefore, compounds 118 and 119 can be studied as new antibiotics [27].
Gracilone (120, Figure 9) was isolated from the methanol extract of Tanacetum gracile. Compound 120 showed moderate antibacterial activity against S. aureus, B. subtilis, E. coli, and P. aeruginosa, with diameters of the growth inhibition zones of 6.70, 14.3, 14.4, and 17.3 mm, respectively, determined by the agar disc diffusion method (50 μg/disk) [54].
3R,8R-Dihydroxygermacr-4(15),9(10)-dien-6S,7S,11RH,12,6-olide (121, Figure 9) is a new sesquiterpenoid lactone extracted from Artemisia sieberi with moderate antibacterial activity against B. subtilis, S. aureus, E. coli, and P. aeruginosa, with an inhibitory area of 6–8 mm [38].
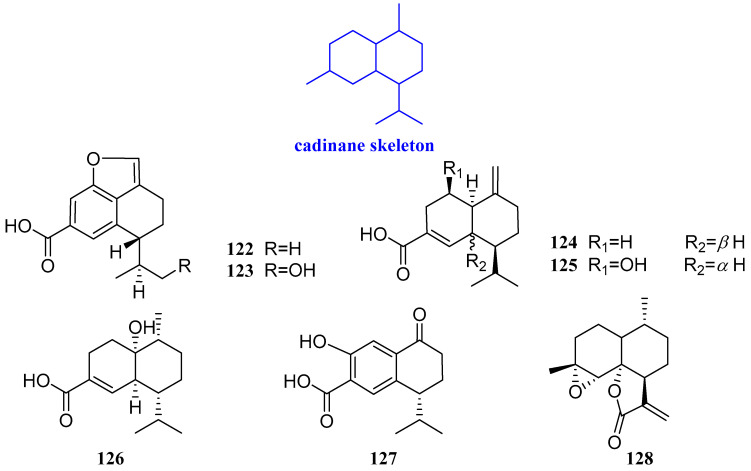
2.8. Cadinanes
Trichocadinins B-G (122–127, Figure 10) were extracted from Trichoderma virens QA-8, an endophytic fungus obtained from the fresh inner tissue of the medicinal plant Artemisia argyi. The antimicrobial activities of compounds 122–127 were evaluated against one human pathogen (E. coli EMBLC-1), 10 marine-derived aquatic bacteria (A. hydrophilia QDIO-1, E. tarda QDIO-2, E. ictarda QDIO-10, M. luteus QDIO-3, P. aeruginosa QDIO-4, V. alginolyticus QDIO-5, Vibrio anguillarum (V. anguillarum) QDIO-6, Vibrio harveyi (V. harveyi) QDIO-7, V. parahemolyticus QDIO-8, and V. vulnificus QDIO-9), and 15 plant-pathogenic fungi (A. solani QDAU-14, B. sorokiniana QDAU-7, C. cornigerum QDAU-8, C. diplodiella QDAU-19, Colletotrichum gloeosporioides (C. gloeosporioides) Penz QDAU-9, F. graminearum QDAU-10, F. oxysporum f. sp. cucumebrium QDAU-16, F. oxysporum f. sp. momordicae QDAU-17, F. oxysporum f. sp. radicis lycopersici QDAU-5, F. solani QDAU-15, G. cingulate QDAU-2, H. maydis QDAU-18, P. digitatum QDAU-11, P. piricola Nose QDAU-12, and V. mali QDAU-13). Chloramphenicol and amphotericin B were used as the positive control against bacteria and fungi, respectively. Compounds 122–127 showed activity against Fusarium oxysporum f.sp. cucumebrium, with MIC values ranging from 1 to 64 μg/mL. Compound 127 had activity against aquatic pathogens E. tarda and V. anguillarum, with MIC values of 1 and 2 μg/mL, respectively, compared with that of the positive control chloramphenicol. Compound 122 exhibited inhibitory activity against the 12 test fungi (except V. anguillarum), with MIC values ranging from 1 to 64 μg/mL [55].
Figure 10.
Structures of cadinane-type sesquiterpenoids 122−128.
Arteannuin B (128, Figure 10) was isolated from Leonurus japonicus with a significant inhibitory effect on E. coli and E. aerogenes with the MIC values of 25 μg/mL and 50 μg/mL, respectively, by assaying the micro-dilution method [56].
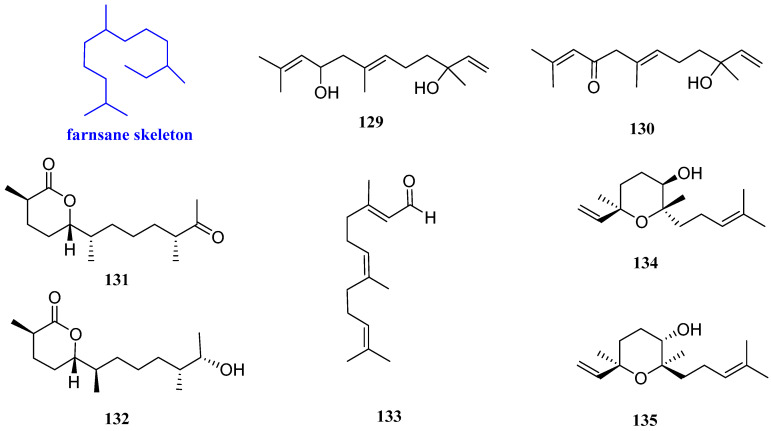
2.9. Farnesanes
9-Hydroxynerolidol and 9-oxonerolidol (129 and 130, Figure 11), possessing chain-like structures, are two farnesane-type sesquiterpenoids isolated from Chiliadenus lopadusanus. The difference between compounds 129 and 130 is that the C-9 hydroxyl group of 129 is oxidized in 130. In the antibacterial experimental assay, 129 exhibited antibacterial activity against Acinetobacter baumannii (A. baumannii) and S. aureus, with MIC values of 150 µg/mL and 75 µg/mL, respectively. Compound 130 exhibited antibacterial activity against A. baumannii and S. aureus, with the same MIC value of 150 µg/mL [57].
Figure 11.
Structures of farnesane-type sesquiterpenoids 129–135.
Chermesiterpenoids B and C (131 and 132, Figure 11) were from the marine red algal-derived fungus Penicillium chermesinum EN-480. Compound 131 showed inhibitory effects on V. anguillarum, Vibrio parahaemolyticus (V. parahaemolyticus), M. luteus, C. gloeosporioides, and human pathogen E. coli with MIC values of 0.5, 16, 64, 32, and 64 µg/mL, respectively. Compound 132 inhibited the aquatic pathogens V. anguillarum, V. parahaemolyticus, M. luteus, and C. gloeosporioides with MIC values of 1, 32, 16, and 64 µg/mL, respectively, while had no effect against E. coli. The positive control amphotericin B had a MIC value of 1.0 µg/mL [58].
Farnesal (133, Figure 11) was isolated from the n-hexane fraction of the crude acetone extract from the leaves of the Australian Plant Eremophila lucida and showed antibacterial activity against S. aureus ATCC 25923 and S. aureus ATCC 29213, with the same MIC value of 65 µg/mL (195 µM) [59].
Rel-(3R,6R,7S)-3,7,11-trimethyl-3,7-epoxy-1,10-dodecadien-6-ol and 6α-hydroxycyclonerolidiol (134 and 135, Figure 11) were isolated from the heartwood of Dalbergia odorifrea T. Chen. Compound 134 was effective against C. albicans, with an inhibition zone diameter of 10.86 mm, and compound 135 exhibited inhibition zone diameters of 9.21 mm against C. albicans and 11.02 mm against S. aureus, respectively [60].
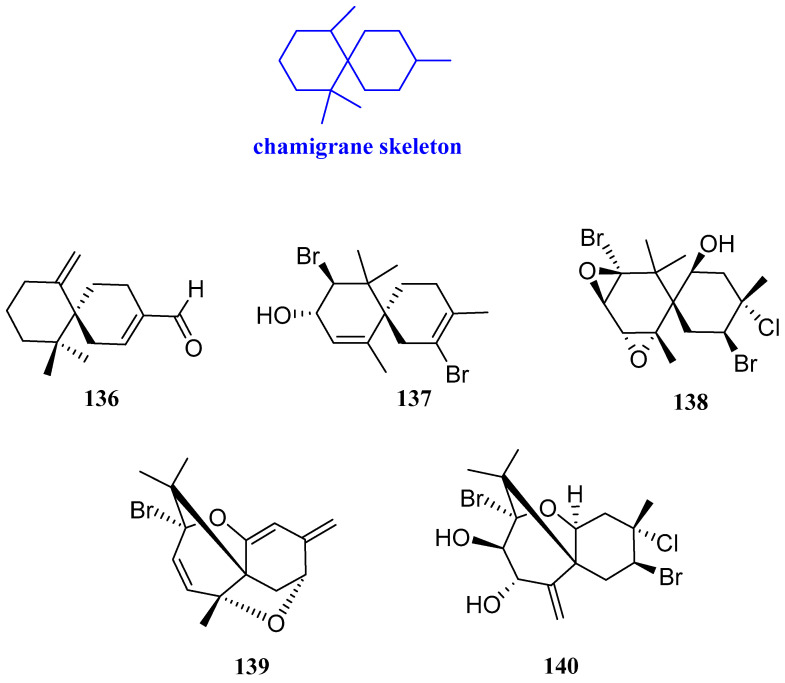
2.10. Chamigranes
The herb of Leonurus japonicus is a type of traditional Chinese medicine that contains a large number of secondary metabolites. It was often used to regulate menstruation and promote blood circulation. One sesquiterpenoid, named chamigrenal (136, Figure 12), was isolated and studied for its antibacterial activities by the microdilution method. The results showed that compound 136 had antibacterial activity against E. coli, E. aerogenes, Macrococcus caseolyticus (M. caseolyticus), S. auricularis, and S. aureus, and the MIC value was in the range from 25 to 200 µg/mL [56]. 2,10β-Dibromochamigra-2,7-dien-9α-ol, prepacifenol epoxide, compositacin N, and pacifenediol (137−140, Figure 12) were isolated from the red alga Laurencia tristicha. Compounds 137−140 had inhibitory activity against S. aureus, M. gypseum, and T. rubrum, with MIC values of 16−118 μg/mL [17].
Figure 12.
Structures of chamigrane-type sesquiterpenoid 136–140.
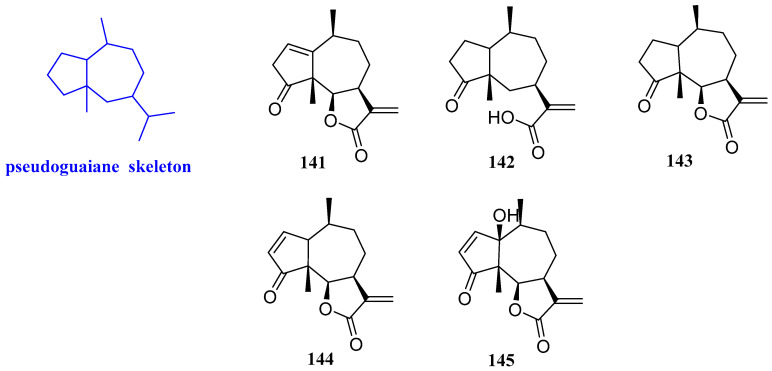
2.11. Pseudoguaiane
Five sesquiterpenoids were isolated from chloroform extract of Ambrosia maritima, including neoambrosin, damsinic acid, damsin, ambrosin, and hymenin (141–145, Figure 13). The antibacterial assays showed that these five sesquiterpenoids had certain antibacterial effects against two plant pathogenic bacteria, Agrobacterium tumefaciens (A. tumefaciens) and E. carotovora, with MIC values ranging from 90 to 520 mg/L. Compound 141 was the most effective against A. tumefaciens and E. carotovora, with MIC values of 150 and 90 mg/L, respectively. In addition, compound 145 caused significant activation of E. carotovora enzymes [61].
Figure 13.
Structures of pseudoguaiane-type sesquiterpenoids 141–145.
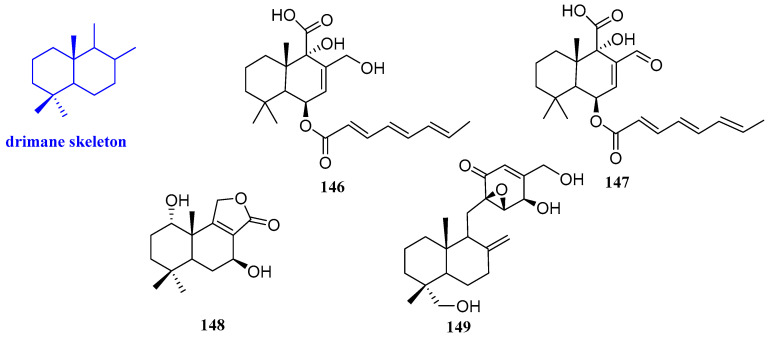
2.12. Drimanes
Two new sesquiterpenoids named ustusoic acid A and B (146 and 147, Figure 14) were isolated from Aspergillus ustus. These compounds had a weak inhibitory effect on vancomycin-resistant Enterococcus faecium (E. faecium) ATCC 700221 and B. subtilis ATCC 49343. Compounds 146 and 147 had a weak effect on B. subtilis ATCC 49343 and vancomycin-resistant E. faecium ATCC 700221, with MIC values ranging from 38 to 128 µg/mL, respectively [62].
Figure 14.
Structures of drimane-type sesquiterpenoids 146−149.
(1S,5S,7S,10S)-Dihydroxyconfertifolin (148, Figure 14) was obtained from Talaromyces purpureogenu residing inside the plant Panax notoginseng, which had an inhibitory effect on E. coli with the MIC value of 25 µM/L [63]. 13-Hydroxylmacrophorin A (149, Figure 14) was isolated from the endophyte Microdiplodia sp. TT-12. Compound 149 had weak activity against R. quercivora, whereas it showed moderate antimicrobial activity against both P. aeruginosa ATCC 15442 and S. aureus NBRC 13276. The results implied that compound 149 is an ingredient that has an antimicrobial against R. quercivora JCM 11526, with an inhibitory area of 12 mm in the culture of Microdiplodia sp. TT-12, which was isolated from the plant hosts [49].
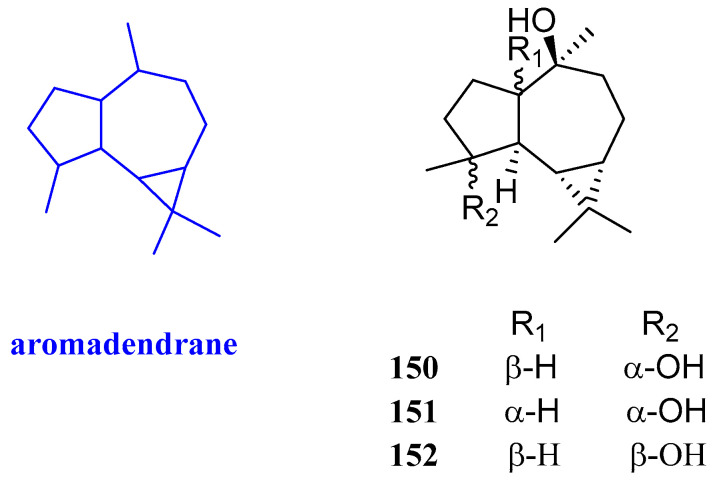
2.13. Aromadendrane
Aromadendrane-4β,10α-diol, aromadendrane-4α,10α-diol, and 1-epimer-aromadendrane-4β,10α-diol (150–152, Figure 15) were isolated from Cassia buds, the immature fruits of Cinnamomum cassia (Lauraceae), and their antibacterial activity was evaluated. Compound 151 showed selective inhibitory activities against S. aureus, with an inhibitory zone diameter of 8 mm, while it had no activity against C. albicans and E. coli. Compound 150 exhibited inhibitory effects against C. albicans, S. aureus, and E. coli, and the inhibitory zone diameters were 10, 7, and 10 mm, respectively. Compound 152 not only inhibited the proliferation of C. albicans but also inhibited the proliferation of S. aureus, with inhibitory zone diameters of 10 and 8 mm, respectively [30].
Figure 15.
Structures of aromadendrane-type sesquiterpenoid 150−152.
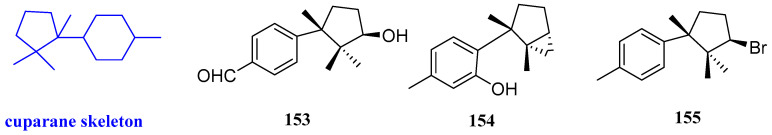
2.14. Cuparanes
Laurencia obtusa lamouroux is a marine species with a variety of biological activities, including antioxidant, antibacterial, and so on. 10-Hydroxycuparaldehyde (153, Figure 16) from L. obtusa lamouroux had good inhibitory activity, with MIC values in the range of 0.08–0.15 mM, for E. coli, Klebsiella pneumoniae (K. pneumoniae), P. mirabilis, P. aeruginosa, E. faecalis, and S. aureus [36].
Figure 16.
Structures of cuparane-type sesquiterpenoids 153–155.
Debromolaurinterol and α-bromocuparane (154 and 155, Figure 16) was isolated from Bornean Laurencia snapeyi. It was found that compounds 154 and 155 had good antibacterial activity against S. typhi, with a MIC/MBC ratio of 2.79 and 2.72, indicating a bactericidal antibiosis [64].
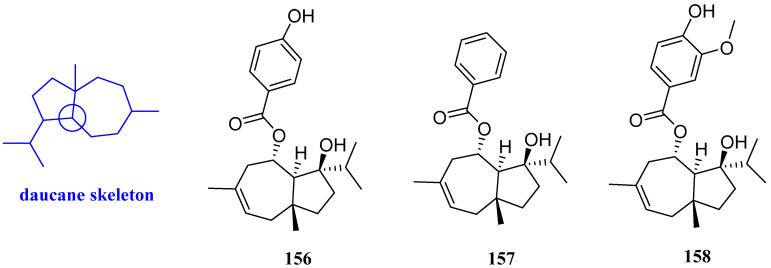
2.15. Daucanes
Jaeschkeanadiol p-hydroxybenzoate (ferutinin), jaeschkeanadiol benzoate (teferidin), and jaeschkeanadiol vanillate (teferin) (156–158, Figure 17) were obtained from the root of Ferula hermonis. Compounds 156–158 possessed antibacterial effects on MRSA, B. subtilis, Mycobacterium tuberculosis (M. tuberculosis), and M. bovis, with MIC values ranging from 0.39 to 8 µg/mL. In this study, positive controls including tetracycline, isoniazid, ciprofloxacin, and chloramphenicol were used; however, no comparison was addressed between the potency of antibiotics and those of compounds 156–158 [65].
Figure 17.
Structures of daucane-type sesquiterpenoids 156–158.
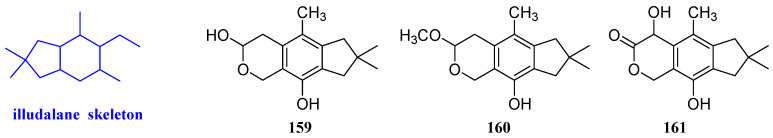
2.16. Illudalanes
The antibacterial activity and cytotoxicity of incarnatin A, incarnatin B, and incarnolactone C (159–161, Figure 18), which were isolated from the mushroom Gloeostereum incarnatum BCC41461, were tested. Compound 161 exhibited anti-B. cereus activity, with a MIC value of 25 μg/mL, while the MIC values of compounds 159 and 160 were both more than 25 μg/mL [66].
Figure 18.
Structures of illudalane-type sesquiterpenoids 159–161.
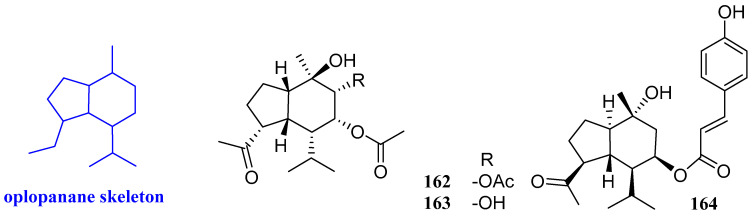
2.17. Oplopananes
Two new sesquiterpenoids were isolated from the ethyl acetate extract of Chimonanthus praecox link, named chimonols A and B (162 and 163, Figure 19). The antimicrobial activities of these two compounds were evaluated and the minimum inhibitory concentrations (MICs) were determined by the broth microdilution method in 96-well culture plates. The results suggested that compounds 162 and 163 had a weak antibacterial effect on S. aureus ATCC 6538 and S. aureus ATCC 25923, and the MIC values were 158.2–223.8 µg/mL. Compounds 162 and 163 were inactive against M. tuberculosis, with MIC values being greater than 250 μg/mL [67].
Figure 19.
Structures of oplopanane-type sesquiterpenoids 162–164.
8-β-p-Coumaroyl-oplopanone (164, Figure 19) was isolated from the ethanol extract of the whole herbs of Pilea cavaleriei. An antibacterial experiment revealed that compound 164 had anti-tuberculosis activity, and the MIC value was 16 µg/mL [68].
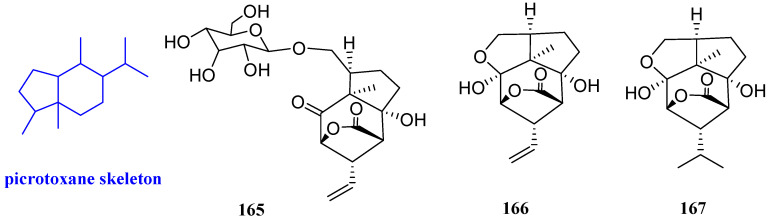
2.18. Picrotoxanes
Three sesquiterpenoids including ramifloside, sapidolide A, and picrotoximaesin (165–167, Figure 20) were isolated from the fruit of Bacurea ramiflora. These three compounds exhibited an inhibitory effect on C. gloeosporioides, and the MIC values were 12.5, 12.5, and 50 μg/mL respectively [69].
Figure 20.
Structures of picrotoxane-type sesquiterpenoids 165–167.
2.19. Rhodolauranes
Rhodolaurenones A–C (168–170, Figure 21) were isolated from Bornean Laurencia majuscula (Harvey) Lucas. Compounds 169 and 170 displayed bactericidal activity against E. coli, S. typhi, and V. cholera, with a MIC value of 100 μg/mL and an MBC value of 250 μg/mL. Compound 168 had a MIC value of 250 μg/mL and an MBC value of 1000 μg/mL, respectively, against E. coli [70].
Figure 21.
Structures of rhodolaurane-type sesquiterpenoids 168–170.
2.20. Others
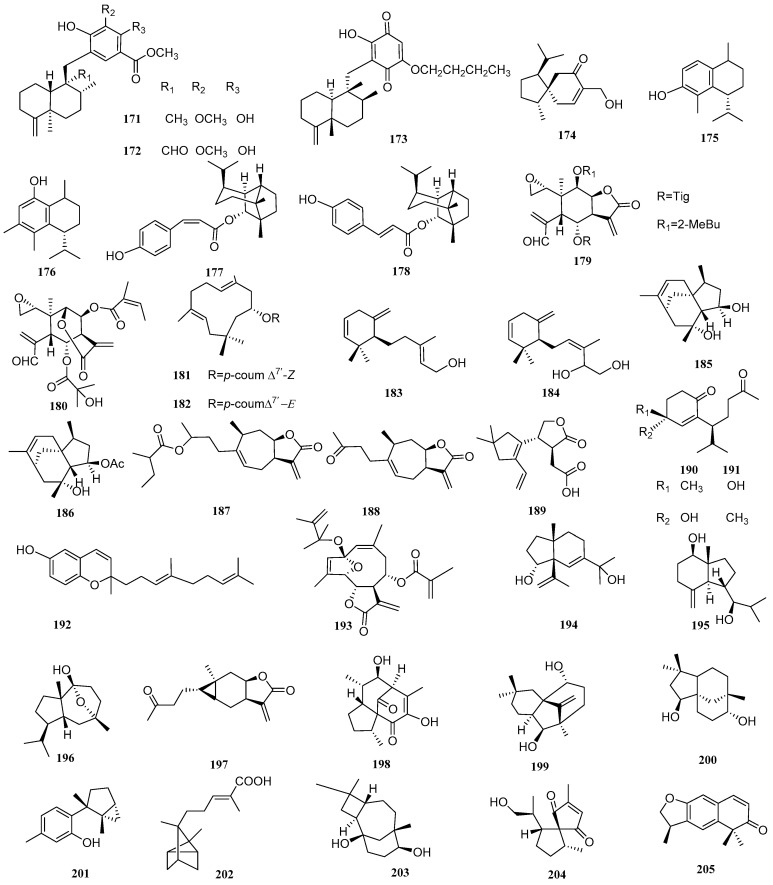
Three sesquiterpenoids were isolated from a Vietnamese marine sponge of Spongia sp. and named as langconols A and C and langcoquinone C (171–173, Figure 22), respectively. The antibacterial assays of these isolates suggested that 171 and 172 possessed significant antibacterial activities against B. subtilis, with MIC values of 12.5 and 25 µM, and 173 also had good inhibitory effects against B. subtilis and S. aureus, with MIC values of 6.25 and 12.5 µM, respectively [71]. Compound 174 (Figure 22), named 4-epi-15-hydroxyacorenone, from Chinese agarwood, could inhibit the proliferation of S. aureus and R. solanacearum, with inhibitory zones of 12.35 and 16.9 mm [48]. Two sesquiterpenoids, dysoxyphenol and 7R,10S-2-hydroxycalamenene (175 and 176, Figure 22), were isolated from the acetone extract of Dysoxylum densiflorum seeds. Both compounds had significant antibacterial properties against B. subtilis (MIC = 28 μM) which were better than those of the positive control amoxicillin (MIC = 34 µM). Compounds 175 and 176 were also evaluated for their antifungal properties against two wood-rotting fungi (brown rot, F. palustris; white rot, T. versicolor) using a zone inhibition assay at two concentrations (0.46 and 4.58 mM). Compound 175 showed the same antifungal effect to both fungi at both concentrations. Compound 176 was able to inhibit the growth of white-rot fungi but not brown-rot fungi at the concentration of 0.46 mM and inhibited both fungi at a higher concentration (4.58 mM) [72].
Figure 22.
Structures of sesquiterpenoids 171–205 of other types.
(1R,2S,5S,6S,7S,10R)-1-O-[(Z)-p-Coumaroyl]-copaborneol and (1R,2S,5S,6S,7S,10R)-1-O-[(E)-p-coumaroyl]-copaborneol (177 and 178, Figure 22) were isolated from Pilea cavaleriei. The antibacterial activities of compounds 177 and 178 were tested, which indicated that these two compounds had moderate antimycobacterial activity against M. tuberculosis H37Rv, with MIC values of 4.84 and 9.83 µg/mL, respectively [73].
New sesquiterpenoid lactones, zinaflorin VI and the δ-elemenolide juniperin (179 and 180, Figure 22), were isolated from Zinnia peruviana L. The MICs of 179 on B. subtilis and S. aureus were 32 and 64 µg/mL, respectively, and the MICs were 4 and 8 µg/mL for compound 180 while the α-Glucosidase inhibition was not active [74].
(1E,5E,8R)-8-O-[(Z)-p-Coumaroyl]humula-1(10),4(5)-dien-8-ol and (1E,5E,8R)-8-O-[(E)-p-coumaroyl]humula-1(10),4(5)-dien-8-ol (181 and 182, Figure 22) were isolated from Pilea cavaleriei. Compounds 181 and 182 showed moderate antimycobacterial activity against M. tuberculosis H37Rv, with MIC values of 3.75 and 7.28 µg/mL, respectively [73]. Genus Laurencia is often studied by researchers, and it has large number of non-secondary metabolites. Two sesquiterpenoids were isolated from Bornean Laurencia snapeyi, including snakeol and snakediol (183 and 184, Figure 22). Researchers tested the antibacterial activity of the two compounds by the microdilution method. The result revealed that compounds 183 and 184 showed strong antibacterial activity against E. coli, with MIC/MBC ratios of 3.02 and 2.76, respectively [64].
Two compounds named penicibilaenes A and B (185 and 186, Figure 22) were obtained from the marine isolate of Penicillium bilaiae MA-267. Both compounds have selective inhibitory effects on C. gloeosporioides, with MIC values of 1.0 and 0.125 μg/mL, respectively [75].
Carpesium macrocephalum has the characteristic of killing fungi. Two sesquiterpenoids, named 4-(2-methybutyryl)-4H-tomentosin and tomentosin (187 and 188, Figure 22), were extracted and isolated from C. macrocephalum. Compounds 187 and 188 inhibited the yeast-to-hyphae morphogenetic transition of C. albicans, with IC50 values of 105.1 and 31.6 μg/mL [33].
A new sesquiterpenoid, named leptosphin B (189, Figure 22), was isolated from the solid fermentation cultures of an endophytic fungus, Leptosphaeria sp. XL026, isolated from the leaves of Panax notoginseng. Compound 189 showed antibacterial activity against B. cereus, with MIC values of 12.5 μg/mL [43]. Chimonols C and D (190 and 191, Figure 22) were extracted from the ethyl acetate extract of Chimonanthus praecox Link. The broth microdilution method was used to test the antibacterial ability. Compound 190 showed activity against S. aureus (ATCC 43300 and ATCC 25923) and C. glabrata (ATCC 2001), with MIC values from 128 to 162 µg/mL. The MIC values of compound 190 against S. aureus (ATCC 25923) and C. glabrata (ATCC 2001) were 183−254 µg/mL. However, both compounds 190 and 191 were inactive against M. tuberculosis, with MIC values over 250 µg/mL [67].
Researchers tested the antibacterial activity and cytotoxicity of (E)-dictyochromenol (192, Figure 22), which was isolated from the brown alga Dictyopteris undulate Holmes. The result found was that compound 192 displayed anti-B. cereus activity, with a MIC value of 1.56 μg/mL [66]. An antibacterial sesquiterpenoid compound from Elephantopus tomentosus, named tomenphantopin H (193, Figure 22), was isolated, possessing an inhibitory effect on S. aureus, with a diameter of the inhibition zone of 14.2 mm, while the diameter of the inhibition zone of the positive control, Kanamycin sulfate, was 32.6 mm [76].
Two compounds, cinnamosim A and 1β,7-dihydroxyl opposite-4(15)-ene (194 and 195, Figure 22), were isolated from Cassia buds, the immature fruits of Cinnamomum cassia (Lauraceae). The antibacterial activities of compounds 194 and 195 were evaluated. The result was that compound 194 and 195 selectively inhibited the proliferation of C. albicans, with inhibitory zone diameters of 11 and 8 mm, respectively, at the concentration of 300 μg/disk. Compound 195 could also inhibit the proliferation of S. aureus, with inhibitory zone diameters of 7 mm at the same concentration [30]. 10-Hydroxy-7,10-epoxysalvialane (196, Figure 22) with antibacterial effects was obtained from Alisma orientale and could inhibit S. aureus, with a MIC value of 100 mg/mL [31].
Carabrone (197, Figure 22) was extracted and isolated from Carpesium macrocephalum. The antibacterial experiment showed that compound 197 had inhibitory activity against C. albicansi and inhibited the yeast-to-hyphae morphogenetic transition through microscopic observation, with an IC50 value of 100.1 μg/mL [33]. Rhodocorane L (198, Figure 22), isolated from the fermentation broth of the basidiomycete Rhodotus palmatus, had medium antifungal ability on N. coryli and R. glutinis, with the same MIC value of 66.7 μg/mL [77]. Antroalbocin A (199, Figure 22) was isolated from Antrodiella albocinnamoea and could inhibit S. aureus, with a MIC value of 169 µM [78].
Clovane-2β,9α-diol (200, Figure 22) was isolated from Eugenia jambolana seeds. It was found that compound 200 had inhibitory activity against S. aureus, with inhibitory zone diameters of 10 mm at the concentration 100 μg/disk. [28]. Debromolaurinterol (201, Figure 22) was isolated from the red algae Laurencia snackeyi. The antibacterial activity of compound 201 was tested by the microdilution method. The result found was that compound 201 showed strong antibacterial activity against S. typhi, with a MIC/MBC ratio of 2.79 [64].
(+)-(E)-α-Santalen-12-oic-acid (202, Figure 22) was isolated from methanol extract of the stem and leaf of Clausena lansium. Compound 202 had weak antibacterial activity against B. cereus, with an IC50 value of 74.6 µM [79]. Caryolane-1,9β-diol (203, Figure 22) was isolated from Cassia buds, the immature fruits of Cinnamomum cassia (Lauraceae). Compound 203 had inhibitory effects on C. albicans, S. aureus, and E. coli, with inhibitory zone diameters of 10, 8.5, and 7 mm, respectively, at the concentration of 300 μg/disk [30]. Rhodocorane K (204, Figure 22) was found to have medium antifungal ability against N. coryli DSM 6981 and R. glutinis DSM 10134, with the same MIC values of 66.7 μg/mL [77]. Variabilone (205, Figure 22) was found in the endophytic fungus Paraconiothyrium variabr and could well inhibit B. subtilis, with an IC50 value of 2.13 µg/mL [80].
3. Mechanisms of Antimicrobial Action by Sesquiterpenoids
The mechanisms of antibiotics against bacteria mainly include affecting cell wall synthesis (β-lactams) and disrupting bacterial membranes, interacting with ribosomal subunits (Tetracycline, Chloramphenicol, Aminoglycosides, etc), disrupting nucleic acid action (Rifampicin, Fluoroquinolones), and interfering with metabolic pathways (Folic acid analogs, sulfonamides) [81]. The corresponding mechanism of antibacterial resistance ranges from accelerating antibiotic efflux through bacterial efflux pumps; alteration of the bacterial porins’ structure, which decreases bacterial permeability to antibiotic influx; and destruction of antibacterial agents by hydrolytic enzymes to alteration of binding sites for antibiotics [82].
The mechanism of sesquiterpenoids against bacteria has not been clearly reported, but it is believed that the microbial cell membranes play an important role. Bacterial subpopulations which are characterized by low metabolism could reduce absorption of antibiotics, especially for the active molecules on the cell wall such as beta-lactams and glycopeptides, making it difficult to treat infections. The mechanism by which the sesquiterpenoids can inhibit the microorganisms involves different modes of action; one which researchers basically agree with is that sesquiterpenoids can destabilize microbial cell membranes. Because the bacterial cell wall is highly lipophilic, it means that a certain lipophilicity is necessary for antibiotics to function [83]. The hydrophobicity of some sesquiterpenoids disturbs the cytoplasmic membrane or compounds in it, such as some classes of proteins, increasing the ionic permeability and causing cytoplasmic extravasation and, as consequence, cellular lysis, as well as interfering with the activity of the respiratory current and energy production. Terpenes isolated from essential oils, such as thymol and carvacrol, may act as permeabilizers of the cell membrane, increasing the entry of antibiotics [54]. Thus, α-bisabolol is possibly responsible for the antibacterial and synergic action when associated with antibiotics. Lipophilic sesquiterpenoids can destroy the membrane and cause ion leakage in the membrane. The results showed that the action mode of β-caryophyllene is to damage the cell membrane and produce non-selective pores, causing the leakage of substances in the cells, and finally causing cell death [84]. Farnesal and farnesol have previously been reported to have antimicrobial activity. Farnesol exerts antibacterial activity by disrupting the cell membrane, and it was also found that it can destroy biofilms of Gram-positive bacteria by reducing biomass [59]. Although the mechanisms responsible for the antibacterial activity of farnesal have not yet been reported, it appears reasonable to hypothesize that farnesal could act in the same way as farnesol, possibly by its hydrophobic nature facilitating insertion into the bacterial phospholipid bilayer membrane and consequent structural disruption. Interestingly, bacteria are able to decrease the concentration of antibiotics in their own cells through the overexpression of efflux pumps. In a mechanistic study, Fazly Bazzaz et al. showed that galbanic acid modulated the resistance in clinical drug-resistant isolates of S. aureus via the inhibition of the efflux pump [85].
The main mechanisms of antifungal effects concern interfering substance transport, yeast-to-hypha transition, host immunity, redox, and others [86]. For instance, polygodial is a sesquiterpenoid dialdehyde that can inhibit fungi. M. V. Castelli et al. carried out experiments using mammalian mitochondrial preparations. The results support the claim that that polygodial mainly plays a role in inhibiting ATP synthesis because the ATP synthesis of phosphorylating submitochondrial Mg+-ATP particles is inhibited at the concentration of polymers similar to the MIC values reported by several yeasts and filamentous fungi [87,88].
Natural products with good inhibitory activity against both bacteria and fungi have broad application prospects in the future development of antifungal drugs. Therefore, it is necessary to clear the mechanisms of action and find antimicrobial targets while exploring new antimicrobial agents.
4. Structure-Activity Relationship
In this review, the structure-activity relationship of compounds with antibacterial activities was analyzed. The compounds with eremophilane, xanthane, lindenane, farnesane, guaiane, penicibilaene, germacrene, daucane, carotene, and illudalane-type skeleton showed relatively strong antibacterial activity (MIC values were lower than 50 μg/mL). Among these types, differences in substituents, different substitution sites, and configuration lead to various degrees of bacteriostatic effect.
Compound 21 had good antibacterial activity against T. rubrum, while compound 22 showed no activity. Apart from C6-OH, compounds 21 and 22 both had a bisabolane skeleton, which may prove the importance of C6-OH in the inhibition of this kind of fungi. Compounds 28–34 all have a similar guaiane skeleton. Comparing the antibacterial activity in pairs, compounds 31, 33, and 34 with C4-OH had strong activity for a variety of pathogenic bacteria, while other compounds without this group had slightly lower antibacterial activity. It seems that C4-OH increased antibacterial activity. In the antibacterial experiment, 71 and 72 are both active against S. aureus, with 71 being more potent than 72. The difference in structures is that the C-9 hydroxyl of 71 was oxidized in 72, which may influence the activity. For farnesanes-type sesquiterpenoids, compound 73 was more active against aquatic and human pathogens than compound 74, but less active against the plant pathogenic fungus, which may be due to the different oxidation degrees of the compounds under C-11. For pseudoguaianes-type sesquiterpenoids, the inhibitory effects of 141 on A. tumefaciens and E. carotovora were stronger than those of 142. The structures of 142 did not have double bonds at C2 and C3, comparing it with 141, which suggested that the double bonds on C-2 and C-3 may increase the antibacterial activity.
5. Discussion
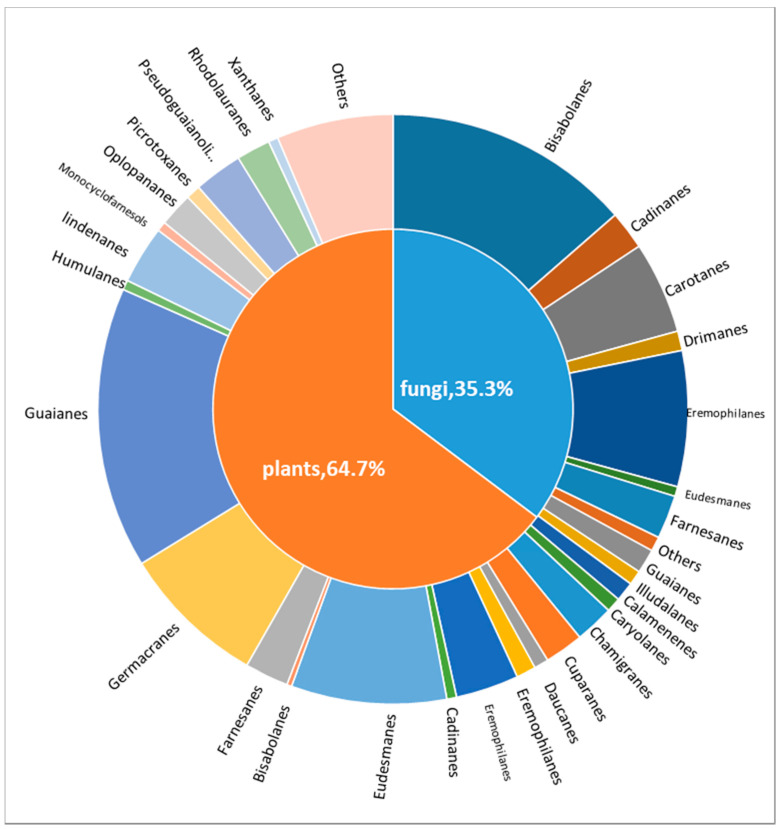
This review shows that a variety of antibacterial sesquiterpenoids were isolated from plants and fungi (64.70% and 35.30%, respectively) (Figure 23). Among them, sesquiterpenoids obtained from plants were mainly distributed in guaiane, eudesmane, germacrane, eremophilane, lindenane, and pseudoguaianolide-type skeletons, with numbers of 58, 32, 30, 19, 13, 12, and 10, respectively. Sesquiterpenoids obtained from fungi were mainly distributed in bisabolane, eremophilane, carotane, farnesane, and cadinane-type skeletons, with numbers of 51, 28, 19, 9, and 8, respectively.
Figure 23.
Skeletal types of antimicrobial compounds of plant and fungal origin.
6. Conclusions
A total of 205 sesquiterpenoids with antibacterial and antifungal activity, which were found and tested from 2012 to 2022, were mainly included in 19 carbon skeleton types, and the number of guaiane sesquiterpenoids was the largest. The names, sources, and chemical structures of 205 sesquiterpenoids are listed in this review. The structure–activity relationship of active compounds is also discussed. According to the data above, we can derive some potential molecules with good antibacterial and antifungal activity. Compound 100 is considered as a potential antimicrobial compound against E. coli, with a MIC of 0.5 µg/mL. Compounds 114 and 134 were most potent against B. licheniformis, with MIC values of 3.1 and 2.3 µg/mL, respectively. Furthermore, compounds 114 and 31 also exhibited an effect on C. albicans, with MICs of 0.26 and 0.21 µg/mL, respectively. Compound 122 showed a striking inhibition of F. oxysporum. F. sp. Cucumebrium and B. sorokiniana, with the same MIC value of 1 µg/mL. As for B. subtilis, compound 180 showed a strong activity (MIC = 4 µg/mL). Compound 192 had a strong effect on B. cereus (MIC = 1.56 µg/mL). The above conclusions were drawn by reviewing large number of sesquiterpenoids and comparing the antimicrobial activities of different structures. The structure-activity relationship plays a key role in modern chemical synthesis and will help people synthesize more effective sesquiterpenoids and use safe natural compounds as antibacterial agents in overcoming bacteria, fungi, and the challenge of drug resistance. These sesquiterpenoids may have the most potential as new natural antibacterial compounds. It is hoped that this review will provide support for the discovery of active drug lead molecules.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom12091271/s1, The Mol. files of sesquiterpenoids with antibacterial and antifungal activity; Table S1: Abbreviations of bacteria and fungi; Table S2: Compounds with antibacterial and antifungal effects. Table S3: Compounds with antibacterial and antifungal effects.
Author Contributions
Conceptualization, H.-Y.L.; data curation, X.-Z.Z. and F.S.; writing-original draft preparation, W.-Q.Y.; writing-review and editing, H.-Y.L.; visualization, T.S. and H.-Y.G.; supervision, L.-M.Z.; project administration, J.Z. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
All authors declare that they have no known or potential competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
This research was funded by Field project Foundation of Ningxia Medical University, Grant Number [XT2020022] and the APC was funded by Field project Foundation of the Ningxia Hui Autonomous Region, Grant Number [2021BEB04060].
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Santajit S., Indrawattana N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016;2016:2475067. doi: 10.1155/2016/2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson T. The Staggering Death Toll of Drug-Resistant Bacteria. Nature. 2022 doi: 10.1038/d41586-022-00228-x. [DOI] [PubMed] [Google Scholar]
- 3.Bongomin F., Gago S., Oladele R.O., Denning D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi. 2017;3:57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rani A., Saini K.C., Bast F., Varjani S., Mehariya S., Bhatia S.K., Sharma N., Funk C. A Review on Microbial Products and Their Perspective Application as Antimicrobial Agents. Biomolecules. 2021;10:1860. doi: 10.3390/biom11121860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martelli G., Giacomini D. Antibacterial and Antioxidant Activities for Natural and Synthetic Dual-Active Compounds. Eur. J. Med. Chem. 2018;5:158. doi: 10.1016/j.ejmech.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 7.White N.J., Hien T.T., Nosten F.H. A Brief History of Qinghaosu. Trends. Parasitol. 2015;31:607–610. doi: 10.1016/j.pt.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White N.J. Qinghaosu (Artemisinin): The Price of Success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 9.Birladeanu L. The Stories of Santonin and Santonic Acid. Angew. Chem. Int. Ed. Engl. 2003;42:1202–1208. doi: 10.1002/anie.200390318. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H.B., Liu C.S., Zheng Q. Development and Application of Anthelminthic Drugs in China. Acta. Trop. 2019;200:105181. doi: 10.1016/j.actatropica.2019.105181. [DOI] [PubMed] [Google Scholar]
- 11.Freund R.R., Gobrecht P., Fischer D., Arndt H.D. Advances in Chemistry and Bioactivity of Parthenolide. Nat. Prod. Rep. 2020;37:541–565. doi: 10.1039/C9NP00049F. [DOI] [PubMed] [Google Scholar]
- 12.Liu X., Bian L., Duan X., Zhuang X., Sui Y., Yang L. Alantolactone: A Sesquiterpene Lactone with Diverse Pharmacological Effects. Chem. Biol. Drug Des. 2021;98:1131–1145. doi: 10.1111/cbdd.13972. [DOI] [PubMed] [Google Scholar]
- 13.Lu J., Xie L., Liu K., Zhang X., Wang X., Dai X., Liang Y.D., Cao Y., Li X.F. Bilobalide: A Review of its Pharmacology, Pharmacokinetics, Toxicity, and Safety. Phytother. Res. 2021;35:6114–6130. doi: 10.1002/ptr.7220. [DOI] [PubMed] [Google Scholar]
- 14.Zhiping P., Dianshi W., Jiandong H., Changgeng Z., Ajing W., Jishuo L. The Effect of Coriaria Lactone on NMDA Receptor Mediated Currents in Rat Hippocampal CA1 Neurons. J. Tongji Med. Univ. 2000;20:6–9. doi: 10.1007/BF02887662. [DOI] [PubMed] [Google Scholar]
- 15.Singh R.D., Mody S.K., Patel H.B., Devi S., Sarvaiya V.N., Patel H.A., Patel B.R. Antimicrobial Drug Discovery: Evident Shifting from Terrestrial to Marine Micro-organisms. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:2322–2327. doi: 10.20546/ijcmas.2017.605.259. [DOI] [Google Scholar]
- 16.Delmondes G.A., Santiago Lemos I.C., Dias D.Q., Cunha G.L.D., Araújo I.M., Barbosa R., Coutinho H.D.M., Felipe C.F.B., Barbosa-Filho J.M., Lima N.T.R., et al. Pharmacological Applications of Farnesol (C15H26O): A Patent Review. Expert. Opin. Ther. Pat. 2020;30:227–234. doi: 10.1080/13543776.2020.1718653. [DOI] [PubMed] [Google Scholar]
- 17.Hu Z.B., Yu X.Q., Wang B., Liu A.H., Zhao T.S., Guo Y.W., Huang H.L., Mao S.C. Structurally Diverse Halosesquiterpenoids from the Red Alga Laurencia Composita Yamada. Fitoterapia. 2020;146:104716. doi: 10.1016/j.fitote.2020.104716. [DOI] [PubMed] [Google Scholar]
- 18.Cheng T., Chepkirui C., Decock C., Matasyoh J.C., Stadler M. Sesquiterpenes from an Eastern African Medicinal Mushroom Belonging to the Genus Sanghuangporus. J. Nat. Prod. 2019;82:1283–1291. doi: 10.1021/acs.jnatprod.8b01086. [DOI] [PubMed] [Google Scholar]
- 19.Wang P., Yu J.H., Zhu K., Wang Y., Cheng Z.Q., Jiang C.S., Dai J.G., Wu J., Zhang H. Phenolic Bisabolane Sesquiterpenoids from a Thai Mangrove Endophytic Fungus, Aspergillus sp. xy02. Fitoterapia. 2018;127:322–327. doi: 10.1016/j.fitote.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Qu H.R., Yang W.W., Zhang X.Q., Lu Z.H., Deng S.H., Guo Z.Y., Cao F., Zou K., Peter P. Antibacterial Bisabolane Sesquiterpenoids and Isocoumarin Derivatives from the Endophytic Fungus Phomopsis Prunorum. Phytochem. Lett. 2020;37:1–4. doi: 10.1016/j.phytol.2020.03.003. [DOI] [Google Scholar]
- 21.Rodrigues F.F.G., Colares A.V., Nonato C.F.A., Galvão-Rodrigues F.F., Mota M.L., Moraes Braga M.F.B., Costa J.G.M.D. In Vitro Antimicrobial Activity of the Essential Oil from Vanillosmopsis Arborea Barker (Asteraceae) and its Major Constituent, α-Bisabolol. Microb. Pathog. 2018;125:144–149. doi: 10.1016/j.micpath.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhary A., Sood S., Kaur P., Kumar N., Thakur A., Gulati A., Singh B. Antifungal Sesquiterpenes from Cedrus Deodara. Planta Med. 2012;78:186–188. doi: 10.1055/s-0031-1280264. [DOI] [PubMed] [Google Scholar]
- 23.Ding Y.H., Wang H.T., Shi S., Meng Y., Feng J.C., Wu H.B. Sesquiterpenoids from Artemisia Vestita and Their Antifeedant and Antifungal Activities. Molecules. 2019;24:3671. doi: 10.3390/molecules24203671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perveen S., Alqahtani J., Orfali R., Aati H.Y., Al-Taweel A.M., Ibrahim T.A., Khan A., Yusufoglu H.S., Abdel-Kader M.S., Taglialatela-Scafati O. Antibacterial and Antifungal Sesquiterpenoids from Aerial Parts of Anvillea Garcinii. Molecules. 2020;25:1730. doi: 10.3390/molecules25071730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shakeri A., Masullo M., Bottone A., Asili J., Emami S.A., Piacente S., Iranshahi M. Sesquiterpene Lactones from Centaurea Rhizantha, C.A. Meyer. Nat. Prod. Res. 2019;33:2016–2023. doi: 10.1080/14786419.2018.1483926. [DOI] [PubMed] [Google Scholar]
- 26.Kudumela R.G., Mazimba O., Masoko P. Isolation and Characterisation of Sesquiterpene Lactones from Schkuhria Pinnata and Their Antibacterial and Anti-inflammatory Activities. S. Afr. J. Bot. 2019;126:340–344. doi: 10.1016/j.sajb.2019.04.002. [DOI] [Google Scholar]
- 27.Cimmino A., Roscetto E., Masi M., Tuzi A., Radjai I., Gahdab C., Paolillo R., Guarino A., Catania M.R., Evidente A. Sesquiterpene Lactones from Cotula Cinerea with Antibiotic Activity against Clinical Isolates of Enterococcus Faecalis. Antibiotics. 2021;10:819. doi: 10.3390/antibiotics10070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F., Liu C., Liu W., Ding Z., Ma H., Seeram N.P., Xu L., Mu Y., Huang X., Li L. New Sesquiterpenoids from Eugenia Jambolana Seeds and Their Anti-microbial Activities. J. Agric. Food Chem. 2017;65:10214–10222. doi: 10.1021/acs.jafc.7b04066. [DOI] [PubMed] [Google Scholar]
- 29.Huang H.F., Zheng C.J., Chen G.Y., Yin W.Q., Huang X., Mo Z.R. Sesquiterpenoids from Curcuma Wenyujin Dreg and Their Biological Activities. Chin. Chem. Lett. 2016;27:1612–1616. doi: 10.1016/j.cclet.2016.03.043. [DOI] [Google Scholar]
- 30.Guoruoluo Y., Zhou H., Zhou J., Zhao H., Aisa H.A., Yao G. Isolation and Characterization of Sesquiterpenoids from Cassia Buds and Their Antimicrobial Activities. J. Agric. Food Chem. 2017;65:5614–5619. doi: 10.1021/acs.jafc.7b01294. [DOI] [PubMed] [Google Scholar]
- 31.Ma Q., Han L., Bi X., Wang X., Mu Y., Guan P., Li L., Huang X. Structures and Biological Activities of the Triterpenoids and Sesquiterpenoids from Alisma Orientale. Phytochemistry. 2016;131:150–157. doi: 10.1016/j.phytochem.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 32.AO W.L.J., Wang Q.H., Qin S., Dan M., Sa R.T.Y., Dai N.Y.T., Tub D.R.S.H.L. The Structural Elucidation and Antimicrobial Activities of Two New Sesquiterpenes from Syringa Pinnatifolia Hemsl. Chin. J. Nat. Med. 2012;10:477–480. doi: 10.1016/S1875-5364(12)60090-9. [DOI] [Google Scholar]
- 33.Xie C., Sun L., Meng L., Wang M., Xu J., Bartlam M., Guo Y. Sesquiterpenes from Carpesium Macrocephalum Inhibit Candida Albicans Biofilm Formation and Dimorphism. Bioorg. Med. Chem. Lett. 2015;25:5409–5411. doi: 10.1016/j.bmcl.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Huang R., Xie X.S., Fang X.W., Ma K.X., Wu S.H. Five New Guaiane Sesquiterpenes from the Endophytic Fungus Xylaria sp. YM 311647 of Azadirachta Indica. Chem. Biodivers. 2015;12:1281–1286. doi: 10.1002/cbdv.201400405. [DOI] [PubMed] [Google Scholar]
- 35.Wang M., Zhao L., Chen K., Shang Y., Wu J., Guo X., Chen Y., Liu H., Tan H., Qiu S.X. Antibacterial Sesquiterpenes from the Stems and Roots of Thuja Sutchuenensis. Bioorg. Chem. 2020;96:103645. doi: 10.1016/j.bioorg.2020.103645. [DOI] [PubMed] [Google Scholar]
- 36.Bawakid N.O., Alarif W.M., Alorfi H.S., Al-Footy K.O., Alburae N.A., Ghandourah M.A., Al-Lihaibi S.S., Abdul-hameed Z.H. Antimicrobial Sesquiterpenoids from Laurencia Obtusa Lamouroux. Open Chem. 2017;15:219–224. doi: 10.1515/chem-2017-0025. [DOI] [Google Scholar]
- 37.Zhang W., Lu X., Huo L., Zhang S., Chen Y., Zou Z., Tan H. Sesquiterpenes and Steroids from an Endophytic Eutypella Scoparia. J. Nat. Prod. 2021;84:1715–1724. doi: 10.1021/acs.jnatprod.0c01167. [DOI] [PubMed] [Google Scholar]
- 38.Mohamed T.A., Hegazy M.F., Aty A.A., Ghabbour H.A., Alsaid M.S., Shahat A.A., Paré P.W. Antimicrobial Sesquiterpene Lactones from Artemisia Sieberi. J. Asian Nat. Prod. Res. 2017;19:1093–1101. doi: 10.1080/10286020.2017.1302939. [DOI] [PubMed] [Google Scholar]
- 39.Li W., Cai C.H., Guo Z.K., Wang H., Zuo W.J., Dong W.H., Mei W.L., Dai H.F. Five New Eudesmane-Type Sesquiterpenoids from Chinese Agarwood Induced by Artificial Holing. Fitoterapia. 2015;100:44–49. doi: 10.1016/j.fitote.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y.H., Wu P.Q., Hu Q.L., Pei Y.J., Qi F.M., Zhang Z.X., Fei D.Q. Cytotoxic and Antibacterial Activities of Iridoids and Sesquiterpenoids from Valeriana Jatamansi. Fitoterapia. 2017;123:73–78. doi: 10.1016/j.fitote.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Li X.H., Yan H., Ni W., Qin X.J., Zhao Q., Ji Z.Q., Liu H.Y. Antifungal Sesquiterpenoids from Chloranthus Japonicus. Phytochem. Lett. 2016;15:199–203. doi: 10.1016/j.phytol.2016.01.005. [DOI] [Google Scholar]
- 42.Wu L., Liao Z., Liu C., Jia H., Sun J. Eremophilane Sesquiterpenes from the Genus Ligularia. Chem. Biodivers. 2016;13:645–671. doi: 10.1002/cbdv.201500169. [DOI] [PubMed] [Google Scholar]
- 43.Chen H.Y., Liu T.K., Shi Q., Yang X.L. Sesquiterpenoids and Diterpenes with Antimicrobial Activity from Leptosphaeria sp. XL026, an Endophytic Fungus in Panax Notoginseng. Fitoterapia. 2019;137:104243. doi: 10.1016/j.fitote.2019.104243. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi T. Antibacterial properties of Nootkatone against Gram-Positive Bacteria. Nat. Prod. Commun. 2019;14:1–5. doi: 10.1177/1934578X19859999. [DOI] [Google Scholar]
- 45.Deshmukh S.K., Dufossé L., Chhipa H., Saxena S., Mahajan G.B., Gupta M.K. Fungal Endophytes: A Potential Source of Antibacterial Compounds. J. Fungi. 2022;8:164. doi: 10.3390/jof8020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang A., Yin R., Zhou Z., Gu G., Dai J., Lai D., Zhou L. Eremophilane-type Sesquiterpenoids from the Endophytic Fungus Rhizopycnis Vagum and Their Antibacterial, Cytotoxic, and Phytotoxic Activities. Front. Chem. 2020;8:596889. doi: 10.3389/fchem.2020.596889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan B., Zhao S., Wang Z., Ye Y., Ahmed R.N., Yan W. Eremophilane Sesquiterpenes and Benzene Derivatives from the Endophyte Microdiplodia sp. WGHS5. Chem. Biodivers. 2021;18:2000949. doi: 10.1002/cbdv.202000949. [DOI] [PubMed] [Google Scholar]
- 48.Li W., Liao G., Dong W.H., Kong F.D., Wang P., Wang H., Mei W.L., Dai H.F. Sesquiterpenoids from Chinese Agarwood Induced by Artificial Holing. Molecules. 2016;21:274. doi: 10.3390/molecules21030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiono Y., Koyama H., Murayama T., Koseki T. New Sesquiterpenes from the Endophyte Microdiplodia sp. TT-12 and Their Antimicrobial Activity. Phytochem. Lett. 2015;14:143–147. doi: 10.1016/j.phytol.2015.10.004. [DOI] [Google Scholar]
- 50.Shi X.S., Song Y.P., Meng L.H., Yang S.Q., Wang D.J., Zhou X.W., Ji N.Y., Wang B.G., Li X.M. Isolation and Characterization of Antibacterial Carotane Sesquiterpenes from Artemisia Argyi Associated Endophytic Trichoderma Virens QA-8. Antibiotics. 2021;10:213. doi: 10.3390/antibiotics10020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X., Wang C., Yang J., Wan D., Lin Q., Yang G., Feng Y. Antimicrobial Sesquiterpenes from the Chinese Medicinal Plant, Chloranthus Angustifolius. Tetrahedron. Lett. 2014;55:5632–5634. doi: 10.1016/j.tetlet.2014.08.052. [DOI] [Google Scholar]
- 52.Xu S., Zhao X., Liu F., Cao Y., Wang B., Wang X., Yin M., Wang Q., Feng X. Crucial Role of Oxidative Stress in Bactericidal Effect of Parthenolide against Xanthomonas Oryzae pv. Oryzae. Pest Manag. Sci. 2018;74:2716–2723. doi: 10.1002/ps.5091. [DOI] [PubMed] [Google Scholar]
- 53.Calzada F., Bautista E., Hidalgo-Figueroa S., García-Hernández N., Velázquez C., Barbosa E., Valdes M., Solares-Pascasio J.I. Understanding the Anti-Diarrhoeal Properties of Incomptines A and B: Antibacterial Activity against Vibrio Cholerae and Its Enterotoxin Inhibition. Pharmaceuticals. 2022;15:196. doi: 10.3390/ph15020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhat G., Masood A., Ganai B.A., Hamza B., Ganie S., Shafi T., Idris A., Shawl A.S., Tantry M.A. Gracilone, A New Sesquiterpene Lactone from Tanacetum Gracile (Tansies) Nat. Prod. Res. 2016;30:2291–2298. doi: 10.1080/14786419.2016.1164701. [DOI] [PubMed] [Google Scholar]
- 55.Shi X.S., Meng L.H., Li X.M., Li X., Wang D.J., Li H.L., Zhou X.W., Wang B.G. Trichocadinins B-G: Antimicrobial Cadinane Sesquiterpenes from Trichoderma Virens QA-8, an Endophytic Fungus Obtained from the Medicinal Plant Artemisia Argyi. J. Nat. Prod. 2019;82:2470–2476. doi: 10.1021/acs.jnatprod.9b00139. [DOI] [PubMed] [Google Scholar]
- 56.Xiong L., Zhou Q.M., Peng C., Xie X.F., Guo L., Li X.H., Liu J., Liu Z.H., Dai O. Sesquiterpenoids from the Herb of Leonurus Japonicus. Molecules. 2013;18:5051–5058. doi: 10.3390/molecules18055051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masi M., Roscetto E., Cimmino A., Catania M.R., Surico G., Evidente A. Farnesane-Type Sesquiterpenoids with Antibiotic Activity from Chiliadenus Lopadusanus. Antibiotics. 2021;10:148. doi: 10.3390/antibiotics10020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu X.Y., Li X.M., Yang S.Q., Liu H., Meng L.H., Wang B.G. Three New Sesquiterpenoids from the Algal-derived Fungus Penicillium Chermesinum EN-480. Mar. Drugs. 2020;8:194. doi: 10.3390/md18040194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biva I.J., Ndi C.P., Semple S.J., Griesser H.J. Antibacterial Performance of Terpenoidsfrom the Australian Plant Eremophila Lucida. Antibiotics. 2019;8:63. doi: 10.3390/antibiotics8020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H., Dong W.H., Zuo W.J., Liu S., Zhong H.M., Mei W.L., Dai H.F. Five New Sesquiterpenoids from Dalbergia Odorifera. Fitoterapia. 2014;95:16–21. doi: 10.1016/j.fitote.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 61.BAdAwy M.E.I., ABdElgAlEIl S.A.M., SugAnuMA T., FujI M. Antibacterial and Biochemical Activity of Pseudoguaianolide Sesquiterpenes Isolated from Ambrosia Maritima against Plant Pathogenic Bacteria. Plant Protect. Sci. 2014;50:64–69. doi: 10.17221/28/2013-PPS. [DOI] [Google Scholar]
- 62.Neuhaus G.F., Loesgen S. Antibacterial Drimane Sesquiterpenes from Aspergillus Ustus. J. Nat. Prod. 2021;84:37–45. doi: 10.1021/acs.jnatprod.0c00910. [DOI] [PubMed] [Google Scholar]
- 63.Zhang B.Y., Ma Y.Y., Guo H., Zhu H.J., Li W. Identification of Two Absolute Configurations of Drimane Sesquiterpenoids from the Endophytic Fungus Talaromyces Purpureogenus of Panax Notoginseng. Chem. J. Chin. Univ. 2017;38:046–1051. [Google Scholar]
- 64.Kamada T., Vairappan C.S. Non-Halogenated New Sesquiterpenes from Bornean Laurencia Snackeyi. Nat. Prod. Res. 2017;1:333–340. doi: 10.1080/14786419.2016.1241996. [DOI] [PubMed] [Google Scholar]
- 65.Ibraheim Z.Z., Abdel-Mageed W.M., Dai H., Guo H., Zhang L., Jaspars M. Antimicrobial Antioxidant Daucane Sesquiterpenes from Ferula Hermonis Boiss. Phytother. Res. 2012;26:579–586. doi: 10.1002/ptr.3609. [DOI] [PubMed] [Google Scholar]
- 66.Bunbamrung N., Intaraudom C., Dramae A., Boonyuen N., Veeranondha S., Rachtawee P., Pittayakhajonwut P. Antimicrobial Activity of Illudalane and Alliacane Sesquiterpenes from the Mushroom Gloeostereum Incarnatum BCC41461. Phytochem. Lett. 2017;20:274–281. doi: 10.1016/j.phytol.2017.05.017. [DOI] [Google Scholar]
- 67.Lou H.Y., Zhang Y., Ma X.P., Jiang S., Wang X.P., Yi P., Liang G.Y., Wu H.M., Feng J., Jin F.Y., et al. Novel Sesquiterpenoids Isolated from Chimonanthus Praecox and Their Antibacterial Activities. Chin. J. Nat. Med. 2018;16:621–627. doi: 10.1016/S1875-5364(18)30100-6. [DOI] [PubMed] [Google Scholar]
- 68.Ren H.C., Zhang J., Liang H. Two New p-Coumaroylated Sesquiterpenoids from Pilea Cavaleriei. J. Asian Nat. Prod. Res. 2018;20:109–116. doi: 10.1080/10286020.2017.1320990. [DOI] [PubMed] [Google Scholar]
- 69.Pan Z.H., Ning D.S., Huang S.S., Wu Y.F., Ding T., Luo L. A New Picrotoxane Sesquiterpene from the Berries of Baccaurea Ramiflora with Antifungal Activity against Colletotrichum Gloeosporioides. Nat. Prod. Res. 2015;29:1323–1327. doi: 10.1080/14786419.2014.999335. [DOI] [PubMed] [Google Scholar]
- 70.Kamada T., Phan C.S., Vairappan C.S. New Anti-Bacterial Halogenated Tricyclic Sesquiterpenes from Bornean Laurencia Majuscula (Harvey) Lucas. Nat. Prod. Res. 2019;33:464–471. doi: 10.1080/14786419.2017.1396593. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen H.M., Ito T., Kurimoto S.I., Ogawa M., Win N.N., Hung V.Q., Nguyen H.T., Kubota T., Kobayashi J., Morita H. New Merosesquiterpenes from a Vietnamese Marine Sponge of Spongia sp. and Their Biological Activities. Bioorg. Med. Chem. Lett. 2017;27:3043–3047. doi: 10.1016/j.bmcl.2017.05.060. [DOI] [PubMed] [Google Scholar]
- 72.Dharmayani N.K.T., Yoshimura T., Hermawati E., Juliawaty L.D., Syah Y.M. Antibacterial and Antifungal Two Phenolic Sesquiterpenes from Dysoxylum densiflorum. Z. Für Nat. C. 2020;75:1–5. doi: 10.1515/znc-2019-0072. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Y., Li L.Y., Yin X., Zhang Q.Y., Liang H., Tu P.F. Sesquiterpenoids from Pilea cavaleriei. Nat. Prod. Res. 2021;35:1537–1543. doi: 10.1080/14786419.2019.1660330. [DOI] [PubMed] [Google Scholar]
- 74.González U., Morales-Jiménez J., Nieto-Camacho A., Martínez M., Maldonado E. Elemenolides from Zinnia Peruviana and Evaluation of Their Antibacterial and α-Glucosidase Inhibitory Activities. Nat. Prod. Res. 2021;35:1977–1984. doi: 10.1080/14786419.2019.1648461. [DOI] [PubMed] [Google Scholar]
- 75.Meng L.H., Li X.M., Liu Y., Wang B.G. Penicibilaenes A and B, Sesquiterpenes with a Tricyclo [6.3.1.01,5]Dodecane Skeleton from the Marine Isolate of Penicillium Bilaiae MA-267. Org. Lett. 2014;16:6052–6055. doi: 10.1021/ol503046u. [DOI] [PubMed] [Google Scholar]
- 76.Wang B., Mei W.L., Zeng Y.B., Guo Z.K., Liu G.D., Dai H.F. A New Sesquiterpene Lactone from Elephantopus Tomentosus. J. Asian Nat. Prod. Res. 2012;14:700–703. doi: 10.1080/10286020.2012.682153. [DOI] [PubMed] [Google Scholar]
- 77.Sandargo B., Michehl M., Stadler M., Surup F. Antifungal Sesquiterpenoids, Rhodocoranes, from Submerged Cultures of the Wrinkled Peach Mushroom, Rhodotus palmatus. J. Nat. Prod. 2020;83:720–724. doi: 10.1021/acs.jnatprod.9b00871. [DOI] [PubMed] [Google Scholar]
- 78.Li W., He J., Feng T., Yang H.X., Ai H.L., Li Z.H., Liu J.K. Antroalbocin A, an Antibacterial Sesquiterpenoid from Higher Fungus Antrodiella albocinnamomea. Org. Lett. 2018;20:8019–8021. doi: 10.1021/acs.orglett.8b03595. [DOI] [PubMed] [Google Scholar]
- 79.Liu X.Y., Fu X.X., Li Y.Y., Xiong Z.H., Li B.T., Peng W.W. The Sesquiterpenes from the Stem and Leaf of Clausena Lansium with Their Potential Antibacterial Activities. Nat. Prod. Res. 2021;35:4887–4893. doi: 10.1080/14786419.2020.1741577. [DOI] [PubMed] [Google Scholar]
- 80.Amand S., Vallet M., Guedon L., Genta-Jouve G., Wien F., Mann S., Dupont J., Prado S., Nay B. A Reactive Eremophilane and Its Antibacterial 2(1H)-Naphthalenone Rearrangement Product, Witnesses of a Microbial Chemical Warfare. Org. Lett. 2017;19:4038–4041. doi: 10.1021/acs.orglett.7b01788. [DOI] [PubMed] [Google Scholar]
- 81.Sultan I., Rahman S., Jan A.T., Siddiqui M.T., Mondal A.H., Haq Q.M.R. Antibiotics, Resistome and Resistance Mechanisms: A Bacterial Perspective. Front. Microbiol. 2018;9:2066. doi: 10.3389/fmicb.2018.02066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jubair N., Rajagopal M., Chinnappan S., Abdullah N.B., Fatima A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR) Evid. -Based Complementary Altern. Med. 2021;2021:3663315. doi: 10.1155/2021/3663315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ivanescu B., Miron A., Corciova A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015:247685. doi: 10.1155/2015/247685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moo C.L., Yang S.K., Osman M.A., Yuswan M.H., Loh J.Y., Lim W.M., Lim S.H., Lai K.S. Antibacterial Activity and Mode of Action of β-caryophyllene on Bacillus cereus. Pol. J. Microbiol. 2020;69:1–6. doi: 10.33073/pjm-2020-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boghrati Z., Iranshahi M. Ferula Species: A Rich Source of Antimicrobial Compounds. J. Herb. Med. 2019;16:100244. doi: 10.1016/j.hermed.2018.10.009. [DOI] [Google Scholar]
- 86.Houšť J., Spížek J., Havlíček V. Antifungal Drugs. Metabolites. 2020;10:106. doi: 10.3390/metabo10030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lyu X., Lee J., Chen W.N. Potential Natural Food Preservatives and their Sustainable Production in Yeast: Terpenoids and Polyphenols. J. Agric. Food. Chem. 2019;67:4397–4417. doi: 10.1021/acs.jafc.8b07141. [DOI] [PubMed] [Google Scholar]
- 88.Castelli M.V., Lodeyro A.F., Malheiros A., Zacchino S.A., Roveri O.A. Inhibition of the Mitochondrial ATP Synthesis by Polygodial, a Naturally Occurring Dialdehyde Unsaturated Sesquiterpene. Biochem. Pharmacol. 2005;70:82–89. doi: 10.1016/j.bcp.2005.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.