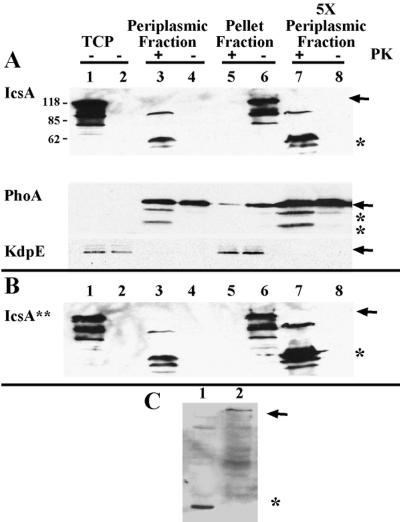
FIG. 5.
Folded state of IcsA in the periplasm. (A) Proteinase K treatment of native IcsA from LDB632 (IcsA) and (B) the LepB-resistant form of IcsA from LDB631 (IcsA∗∗). Shown are periplasmic proteins (lanes 3, 4, 7, and 8), and pellet fractions, which contain cytoplasmic and inner and outer membrane fractions (lanes 5 and 6), from LDB631 and LDB632 in the presence (lanes 3, 5, and 7) or absence (lanes 4, 6, and 8) of proteinase K. Western blot analysis was done with polyclonal antibodies to IcsA, PhoA, or KdpE. Protein (10 μg) was loaded into each of lanes 1 to 6; 50 μg of protein was loaded into each of lanes 7 and 8. Lane 1, total cellular protein (TCP) of 2457T (wild type); lane 2, total cellular protein of MBG283 (icsA); lanes 3 to 8, LDB631 (IcsA∗∗) or LDB632 (IcsA). Arrows indicate full-length proteins. Asterisks indicate proteins truncated by proteinase K treatment. Results from one representative experiment of three are shown. (C) Western blot analysis of pellet fraction of proteins isolated from LDB631 in the presence (lane 1) or absence (lane 2) of proteinase K, using polyclonal antibodies to the β domain of IcsA. Arrow indicates full-length IcsA. Asterisk indicates protein truncated by proteinase K treatment.