Figure 3. Adverse outcomes at short‐term follow‐up (≤30 days). 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 .
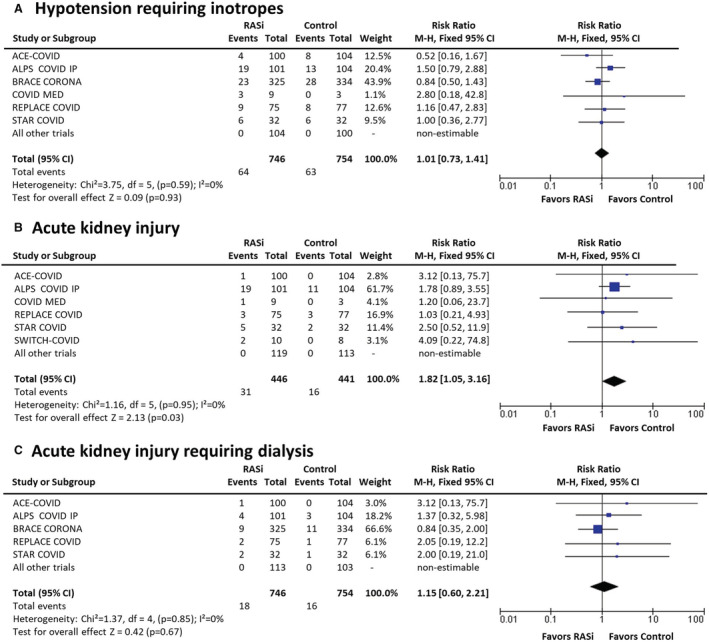
ACEI‐COVID, the stopping ace‐inhibitors in COVID‐19 trial; ALPS‐COVID IP, angiotensin receptor blocker based lung protective strategy for COVID‐19 inpatient trial; ALPS‐COVID OP, angiotensin receptor blocker based lung protective strategy for COVID‐19 outpatient trial; BRACE CORONA, blockers of angiotensin receptor and angiotensin‐converting enzyme inhibitors suspension in hospitalized patients with coronavirus infection; COVERAGE‐France, randomized trial to evaluate the safety and efficacy of outpatient treatments to reduce the risk of worsening in individuals with COVID‐19 with risk factors; COVID MED, comparison of therapeutics for hospitalized patients infected with SARS‐CoV‐2; M‐H indicates Mantel–Haenszel; PRAETORIAN‐COVID, randomised clinical trial with valsartan for prevention of acute respiratory distress syndrome in hospitalised patients with SARS‐COV‐2 infection disease; RAAS‐COVID, renin‐angiotensin aldosterone system inhibitors in COVID‐19 trial; RASi, renin‐angiotensin system inhibitors; REPLACE COVID, the randomized elimination or prolongation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in coronavirus disease 2019; STAR‐COVID, telmisartan in respiratory failure due to COVID‐19; and SWITCH‐COVID, switch of renin‐angiotensin system inhibitors in patients with COVID‐19.
